Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Addressing Barriers to Chronic Obstructive Pulmonary Disease (COPD) Care: Three Innovative Evidence-Based Approaches: A Review
Authors Siu DCH , Gafni-Lachter L
Received 31 October 2023
Accepted for publication 17 January 2024
Published 1 February 2024 Volume 2024:19 Pages 331—341
DOI https://doi.org/10.2147/COPD.S426050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jill Ohar
Damian Chi Hong Siu, Liat Gafni-Lachter
Boston University, Sargent College of Health and Rehabilitation Sciences, Boston, MA, USA
Correspondence: Damian Chi Hong Siu, Graduate, Post-professional Occupational Therapy Doctorate, Boston University, Sargent College of Health and Rehabilitation Sciences, 635 Commonwealth Ave, Boston, MA, 02215, USA, Email [email protected]
Abstract: Chronic obstructive pulmonary disease (COPD) is a preventable yet widespread and profoundly debilitating respiratory condition, exerting substantial personal and global health ramifications alongside significant economic implications. The first objective of this literature review was to identify reviews the barriers to optimal COPD care, categorizing them into personal patient factors, professional awareness and knowledge, patient-professional relationships, and healthcare service models, including access to care that significantly impacts the quality of COPD management. The second objective was to introduce three approaches for enhancing COPD care outcomes: Self-Management Educational Programs, Health Qigong, and Telehealth service provision, each demonstrating positive effects on COPD patients’ health status. These evidence-based interventions offer promising avenues for enhancing COPD care and patient outcomes. Integrating these approaches into comprehensive COPD management strategies holds potential for improving the well-being and quality of life of individuals living with this chronic condition.
Keywords: pulmonary disease, chronic obstructive, health qigong, telehealth, self-management education
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable respiratory disease that is the third leading global cause of death, accounting for 3.2 million people annually.1 It is a chronic condition characterized by airflow limitation with a progressive decline in lung function over time, especially in smokers, that requires careful ongoing management.2 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines assess patients according to their level of symptoms and previous history of exacerbations. The severity of COPD is based on the classification of airflow limitation by pulmonary function testing, with a post-bronchodilator ratio of the forced expiratory volume in the first second to the forced vital capacity of the lungs (FEV1/FVC) ratio of <0.7.3 Treating COPD has significant financial impacts on health care systems; it accounts for approximately 4% of all public hospital annual admissions in Hong Kong4 and is a leading cause of hospital readmissions.5–8 As a common diagnosis associated with hospital readmission within 30 days, COPD greatly impacts hospital stays and healthcare expenses worldwide.4,5,9 People with COPD deal with reduced lung function, limited energy expenditure, and functional capacity to meet exertional demands. Increased shortness of breath and dyspnea often induce fear of participating in daily activities. People may become progressively sedentary as their activity tolerance deteriorates over time. Common comorbidities include cardiovascular disease, osteoporosis, muscle wasting, underweight and obesity, metabolic disorders, and anxiety and depression. In addition, the signs and symptoms of COPD are highly associated with the person’s adherence to a health regimen, respiratory hygiene, cough etiquette, and environment and weather conditions.10–12
Suboptimal management of COPD reduces patients’ quality of life and increases their risk of acute exacerbation of COPD, leading to hospital admission and readmission – and increased risk of mortality. An increasing number of severity of exacerbations were associated with increasing risk of subsequent exacerbation, all-cause mortality and COPD-related mortality.13 Increased prevalence of bronchiectasis was reported, which is associated with more frequent and severe exacerbations, impaired quality of life and possibly reduced survival.14 High morbidity and mortality rates resulting from poor COPD care, high crude fatality rate of COVID-19 (7.4%), 1.45 times more likely with severe complications with current smokers.15 Bourbeau and Bartlett highlighted patients with comorbid COPD and heart failure are at a 1.61 times higher risk of all-cause mortality and 2.01 times for COPD-related hospitalization that COPD patients without heart failure. The adjusted risk ratio was 1.01 for 30 days and 1.11 for 1 year of COPD rehospitalization.16 Hansel, McCormack and Kim conducted a critical review and suggested the prevalence of COPD-related readmission varied from 2.6 to 82.2% at 30 days and 25.0–87.0% at 12 months post-discharge.17 Furthermore, non-adherence to COPD medication, incorrect inhaler technique, and/or selection of appropriate inhaler device worsens clinical and economic outcomes.17,18 Peak Inspiration Flow (PIF) was one of the functional parameters to evaluate the adherence to treatment or errors in inhalation technique.19
Best practices in COPD care call for a multidisciplinary team that includes collaboration among occupational therapists, physical therapists, and nurses with pulmonary rehabilitation experts. A 2016 Lancet review2 explains that optimal COPD care includes appropriate drug treatment, nutrition counseling and modulation, physical activity coaching, energy conservation, exercise training, self-management, and psychological counseling. The presence of co-occurring, chronic, noncommunicable diseases and other physical and psychological manifestations must be addressed to characterize and manage the needs of individual patients with COPD.2 The Global Initiative for Chronic Obstructive Lung Disease strategy document recommended guidelines for managing comorbidities, suggesting it should be personalized for the individual.3 Multiple components of interventions would enhance COPD management.20
This article aimed to first examine and understand barriers to optimal and effective COPD care, and second, to review three emerging innovative approaches that are effective in augmenting the health of those with COPD.
Method
This review was conducted as part of a doctoral project to develop effective COPD interventions.21 We used a modified umbrella review approach22 to summarize and evaluate published systematic reviews related to barriers to COPD care and outcomes and the FEV1 means to remediate this gap. Reviews were identified through an exhaustive search of electronic databases, including PubMed, MEDLINE, EMBASE, CINAHL Plus with Full Text Comprehensive, and Cochrane Database of Systematic Reviews. Limitations were set to January 2012- December 2022 to obtain the latest evidence from reviews, systematic reviews, or meta-analyses. Key terms were entered in various combinations with multiple Boolean operators. They included COPD, chronic obstructive pulmonary disease, with keywords for the best COPD care and barriers and evidence-based solutions. Following the database search, the investigators conducted hand searches (ie, manual searching and scanning print journals for review articles) of reference lists within each article. The inclusion criteria included a peer-reviewed publication, English language, clinical diagnosis of COPD, eg post-bronchodilation FEV1/FVC<0.7, and age above 40. Interventions targeted at patients with multiple comorbidities outside of COPD were excluded.
Results
The literature search yielded a total of 56 resources for this paper: 44 were systematic reviews and meta-analyses, and 13 were literature reviews. The evidence regarding barriers to optimal COPD care covered various aspects: personal patient factors (mentioned in one systematic review23 and five literature reviews);24–28 the impact of professionals’ knowledge and behaviors on care quality (discussed in one literature review);26 Patient-Professional Relationship (addressed in one systematic review and five literature reviews); Health Care Service Models (covered in five meta-analyses/systematic reviews20,29–32 and one literature review);33 and Access to COPD Care (explored in four meta-analyses/systematic reviews34–37 and one literature review).26
Regarding innovative evidence-based approaches to COPD care, the evidence encompassed Self-Management Educational Programs (featured in nine meta-analyses/systematic reviews38–46 and four literature reviews),47–50 Health Qigong (included in ten meta-analyses/systematic reviews),51–60 and Telehealth (highlighted in seven meta-analyses/systematic reviews29–31,61–64 and one literature review).65
Barriers to COPD Care
The literature search revealed multiple barriers that were organized into three categories: personal patient factors, professionals’ knowledge and awareness of COPD management, patient-professional relationships and psychological factors, healthcare service model and access to care.
Personal Patient Factors
Evidence demonstrated that despite a common notion that adherence is related to personal patient factors, it is a multifactorial phenomenon influenced by the patient, clinician, and society.10,27 A meta-analysis of 50 years of adherence research in chronic diseases demonstrated that non-adherence behaviors are not associated with age, gender, educational level and socioeconomic status.66,67 However, the six dimensions of medication nonadherence were identified and outlined by the World Health Organization.25,28 These are: Social/economic (eg, illiteracy, transportation); Health system (eg, insurance coverage), dissatisfaction with the treating physician, and limited interaction between clinicians and patients; Therapy-related (eg, polypharmacy); Condition-related (eg, fragility), the severity of the disease, and concern about the medicine’s harmful effects; Patient-related (eg, self-efficacy, knowledge); and Informal caregiver (eg, overprotect Clinical inertia is broadly defined as “recognizing the problem but failing to act”.68 It includes patients’ nonadherence to the prescribed treatment, therapeutic inertia (providers fail to initiate medications or intensify treatment), and inappropriate therapy.69 Reasons for clinical inertia in managing nonadherence can be classified into provider, patient, and system factors. According to systematic reviews of medication nonadherence conducted among COPD patients, the rates of nonadherence ranged from 22% to 93%. Most of the studies came from high-income countries.23,26
Low social and economic status and inadequate family and social support negatively affect most health conditions, including COPD.70,71 People with COPD who live in remote places far from a health care centre and do not have access to transportation may have limited ability to receive services.70,71
On the other hand, people’s attitude, including perceptions of COPD severity, influences their willingness to participate in the demanding health regimens required to manage COPD.27 The severity of COPD cannot be merely documented by subjective measures such as self-reported dyspnea at rest or with exertion, chronic cough with or without sputum production, or a history of wheezing: COPD severity must be regularly assessed for airflow obstruction by spirometry (eg FEV1 and FVC) and combined with results from various subjective and objective evaluations such as a Modified Medical Research Council Dyspnea Scale (mMRC) and the COPD Assessment Test (CAT).72 In addition, psychological issues, such as anxiety, social isolation, inadequate social support and depression, are important factors contributing to nonadherence.23–25
Finally, the presence of an informal caregiver is an essential factor influencing COPD care.28,73 The company of an informal caregiver provides practical help and emotional support in COPD management. However, informal caregiving may lead to anxiety, depression, social isolation, and a changed relationship with the patient. Nevertheless, the research suggested that overprotective caregivers can make patients more dependent.
Five literature reviews provided qualitative evidence about the effect of an informal caregiver’s presence, dimensions, and factors associated with nonadherence.24–28 It used informal or subjective methods to collect and interpret studies, so the findings may be biased.
Based on the evidence, it was proposed solutions to medication nonadherence, including addressing the patient’s belief in the medication, better understanding of their disease and drug therapy, confidence in the health care professionals’ expertise, reducing the number of inhaler devices and the dosing regimen, repeating instructions, and reinforcing the potential for better quality of life and satisfaction with their inhaler devices and less frequent exacerbation.23 A coordinated, multifaceted approach was suggested in improving COPD care and adherence, including the use of health informatics, changes in provider workflow, application of objective and performance measures, stimulating patient empowerment, and education and training.26,27 It also suggested mobile telephone technology and electronic monitoring and devices as alternative solutions.24,27 Informal caregivers should be involved because the solutions could improve the quality of life for patients and their informal caregivers and save health care costs.
Professional Awareness and Knowledge of COPD Management
Research findings support the proposition that professionals’ awareness, knowledge, and resulting behaviors are key to improving best-practice care and management of COPD.26,74 The positive doctor–patient relationship, including listening and understanding, can also help patients adhere more to treatment, improve their lifestyle, resume activities interrupted because of failing health, and improve their quality of life.
It was suggested that provider factors may affect the appropriate management of COPD, including understanding and attitudes toward the disease and awareness of guidelines.26
The evidence presented here supports the connection between professionals’ knowledge and behaviors and COPD care as it appears in the explanatory model. Although the quality of the literature review was lower, the evidence highlighted a multifaceted approach to enhancing clinical inertia in managing COPD was recommended.26 The review evidence supports the influence of professionals’ knowledge and behavior factors underpinned in the explanatory model. Smoking cessation can effectively reduce the risk of death, alleviate respiratory symptoms, and decrease the frequency of acute exacerbations among patients with COPD.75 Theory-based smoking cessation intervention has a productive impact on motivating patients to quit smoking, improving their lung function and quality of life. Common education approach for patients with COPD and smoking history including 5A approach (Ask, Advise, Assess, Assist, and Arrange), 5R approach (Relevance, Risks, Rewards, Roadblocks, and Repetition), and the motivational interviews.3
Based on the evidence, solutions should address a multifaceted approach,26 a positive doctor-patient relationship, local health system and delivery, direct costs to patients, the causal role of smoking in COPD,76 and adoption of guidelines in COPD treatment choice.74
Patient–Professional Relationship and Psychological Factors
A positive relationship, including listening and understanding, was key to improving clinical care and management of COPD. The relationship quality impacted the adherence and effectiveness of therapies and resumption of activities. It is also strictly linked to successful smoking cessation, achieving a “therapeutic alliance”, and commitment to pharmacological or rehabilitation treatments. A productive relationship helps patients adhere more to treatment, improve their lifestyle, resume activities interrupted because of failing health, and improve their quality of life. Furthermore, psychological issues such as anxiety, social isolation, inadequate social support and depression were leading causes of medication nonadherence and poor COPD management.23–25
Health Care Service Model and Access to COPD Care
The service model of health care management and administration can act as a barrier to facilitating COPD care. Current infection control measures guide the setup of service provision locations. Places that are not up to infection control standards cannot provide care, which inhibits access to care. Inconsistent COPD care and referral pathways; fragmented COPD services, interventions, and resources; multidisciplinary teams not working; and miscommunication were the identified barriers under the catchment of current service provision.77
Multiple studies confirmed that integrated disease management (IDM) programs contributed to efficient quality care.6,26,33–35,39,78 These programs included different health care professionals, such as family and respiratory physicians, nurses, physiotherapists, and occupational therapists.
A meta-analysis summarized 52 worldwide randomized controlled trials on IDM.20 Those authors indicated various components of care that enhanced COPD management, including organizational, professional, patient-directed, and financial interventions. Meanwhile, services promoted regular meetings with family physicians and health professionals and referred all COPD patients to attend the self-management program as mandatory. These types of services facilitate effective care and reduce the risk of acute exacerbation.78 In addition, introducing telehealth services and normalizing COPD self-management into routine practice would enhance coordinated COPD primary and secondary management worldwide.
Findings of several systematic reviews29,34,35,37 confirmed the influence of access to care on COPD care and identified sources that influence access to care. There are three factors affecting access to care for COPD patients—provider, system, and patients—and gave examples, such as appropriate management of COPD service, insurance coverage, and access to proper puff medications.26 Results of the systematic reviews provided evidence of a myriad of factors that influence access to care, including the location of service provision, lack of transport service, wait time, burden of illness, health system resources, fragmented care with lack of communication, modalities of access to the services, and sanitary facilities with physical and architectural barriers.29,34,35
Innovative Evidence-Based Approaches to COPD Care
Effective evidence-based solutions should address a multifaceted, innovative delivery format supporting patients attending training in primary care,26,34 addressing personal and health care system environment barriers in access to care29,35 and patient and caregiver values.37 The evidence is organized according to three key evidence ingredients: self-management education programs, health qigong and telehealth for effective COPD care.
Self-Management Educational Programs Influence the Health Status of Patients with COPD
Self-Management Education program have been shown to have positive health outcomes, especially in primary care settings.35,43–52,79,80 Furthermore, COPD self-management interventions were deemed safe and unlikely to cause harm.38
Three Self-Management Intervention models of care across the continuum of exacerbations were summarized: (a) chronic care and Self-Management Interventions with an action plan, (b) domiciliary care for severe exacerbation and its impact on readmission prevention, and (c) a discharge care bundle for management beyond the acute exacerbation.48 All three interventions aim to improve quality outcomes, enhance patient well-being, and reduce exacerbation complications such as hospital admissions/readmissions. Interventions should focus on controlling costs by avoiding hospitalizations. The authors recommended that future models of care should be personalized—providing patient education aimed at behavioral changes, identifying and treating comorbidities, and including outcomes that measure the quality of care. A COPD written action plan for adherence can be further used and enhanced with telehealth technologies in a specialized clinic experienced in COPD self-management. A complete feedback loop process should be implemented to constantly assess whether the desired outcomes are being achieved for a patient with personalized self-management in COPD.47,79 The program durations typically ranged from 1 month to at least 2 years.40
Telemedicine can be an adjunct to self-management approaches, assisting proper health care coaching.48 It was recommended the following keys to success in COPD self-management plans:49
- better education for healthcare professionals on disease management and consultation skills;
- new targets and priorities for patient-focused outcomes;
- skills-gap audits to identify barriers to self-management;
- best-practice sharing within primary care networks and ongoing professional development;
- enhanced initial consultations to establish optimal self-management from the outset; and
- negotiate and share self-management plans at the point of diagnosis.
The content of the self-management program was suggested, including intervention strategies and common assessment and training tools.39 The self-management program’s content mostly included anatomical structures of respiratory ways and lungs, pathophysiology, common symptoms, progress and disease stages, conventional medications, exacerbation management, daily exercises, and breathing retraining. It also focused on energy conservation techniques, lifestyle changes, smoking cessation, coping with anxiety and stress, training family caregivers, and nutritional issues. It was recommended that the self-management program identify support that helps people self-manage and adapt to life with mild/moderate COPD,40 reducing the impact of this slowly progressive condition in primary or community settings. Self-management support preferences were identified as helping people engage with self-management support and facilitating better self-management, including types of support, support relationships, and accessibility.43
Substantial evidence concluded that Self-Management Interventions with a COPD exacerbation action plan were associated with improved health-related quality of life and reduced emergency department visits over 12 months.38,41,42,44,45 It suggested that effective interventions include iterative interactions between participants and health care professionals using behavioral change techniques. Applying self-management interventions elicits participants’ motivation, confidence, and competence to adapt their health behaviors positively and cultivate better coping skills to manage their disease.38 Another systematic review and meta-analysis were conducted, and intervention descriptions for behavioral change techniques were coded, addressing (a) symptoms, (b) physical activity, and (c) mental health.42 Self-management interventions should target not only symptom management but also mental health issues, including social support and reducing negative emotions.
Health literacy should be emphasized in training.46 It drives the self-management program and significantly improves patients’ disease knowledge and physical activity levels. A scoping review was conducted and identified four main components in COPD patients’ self-management programs: the initiation stage of the intervention, educational sessions, support, and monitoring methods. The common characteristics of the intervention included:50
- Initiation intervention sessions could have a positive impact because they test the patients’ motivation for the intervention; they could contribute to better outcomes in self-management programs.
- Action plans engage patients in managing their disease.
- Educational materials helped patients in the self-management process.
- Phone calls are intended to motivate, engage, and accompany patients throughout the intervention.
It was recommended that self-management interventions with e-health blended with face-to-face interventions reduce the disease burden with significant positive effects on various health-related outcomes.44 The rate of 30-day hospital readmission and the number of hospital admissions in the past 12 months should be compared due to acute exacerbation for high-risk patients with COPD to monitor the quality of care for COPD.48
Health Qigong for Patients with COPD
Health Qigong is a “mind-body exercise”80 that enforces a state of relaxed mind rhythmic, deep, and slow breathing, sometimes with the use of the diaphragm; and motion coordinated with breathing. Health qigong is an integration for a state of balance/homeostasis by regulating the body, breathing, and mind. It is an innovative way to promote self-management and positive health outcomes for COPD patients.51–55 The findings confirmed the positive influence of HQG on the health status of patients with COPD care.
Regularly practicing HQG was found to promote the health status of patients with COPD.53 Results of several meta-analyses provided evidence of the contributions of HQG to reducing anxiety and depression.53,57 Substantial evidence showed that HQG practice enhanced lung function,53–55,58–60 minimized the perceived severity of dyspnea,58,59 and promoted physical fitness.51,53–55,58–60 The improved physical and psychosocial health further uplifted the patient’s immunity81,82 and quality of life (QOL).51,53–55,58,59
Telehealth for Effective COPD Care
Telehealth is a broad term referring to the delivery of healthcare services where patients and healthcare providers are separated by distance. It relies on technology to exchange information with healthcare providers and can be asynchronous or synchronous.83 A systematic review concluded that common telehealth interventions for patients with COPD included self-management programs via telemonitoring or self-management programs combined with other interventions (eg exercise, mobile apps for pulmonary rehabilitation, or home care).84 In delivering the telehealth interventions, it should be aware of sociodemographic and intervention-related factors in promoting acceptance and minimizing dropout rate. Evidence suggests that telerehabilitation can deliver self-management programs effectively, increasing accessibility and adherence.30,62,64,65
Evidence from the intervention programs showed that primary pulmonary rehabilitation, or maintenance rehabilitation, delivered via telerehabilitation or home-based exercise therapy provided using advanced telehealth technology for people with COPD, achieved outcomes similar to traditional center-based pulmonary rehabilitation. No safety issues were identified.29,30,61 Home-based telehealth pulmonary rehabilitation had similar effects to outpatient pulmonary rehabilitation programs and more significant results than usual care for people with COPD.63 Telerehabilitation promoted the program completion rate29,30 and was beneficial as an additional health resource, depending on individual needs based on professional assessment.31
Multicomponent telerehabilitation interventions with asynchronous remote monitoring were not better than usual care but provided short-term benefits for quality of life and resulted in fewer hospital readmissions for any cause.31 The introduction of smart technology added a significant positive effect on activity level, self-management, and subsequent health-related quality of life in terms of symptoms and health status compared to participants who received face-to-face, digital, and/or written support for self-management of COPD.62 However, the improvement may not be sustained over a long duration without continued intervention.
A standardized outcome-reporting framework for digital health interventions in COPD self-management was recommended.64 Monitoring devices such as pulse oximeters and pedometers linked to mobile apps can facilitate activity monitoring and compliance with the action plan.
A meta-analysis31 suggested that most telehealth interventions for patients with COPD range from 13 to 52 weeks. Significant heterogeneity of health qigong types was included in the trials of pulmonary rehabilitation programs. To produce a substantial health effect after regular practice of health qigong, most interventions ranged from 6 to 12 weeks, with each session lasting at least 30 min.51,54,56,58,60
Discussion
The aim of this literature review was twofold: first, to assess the main obstacles to providing optimal care for COPD, and second, to explore three approaches for improving COPD care. The evidence collected was organized into an explanatory model, shown in Figure 1. The complexities involved in managing COPD include patient personal factors, professionals’ knowledge and behavior, and healthcare service models, as well as the mediating role of access to care. All these significantly contribute to poorer health outcomes for individuals. Inadequate COPD management heightens their risk of acute exacerbations. Nevertheless, the evidence points to the potential contribution of three approaches: telehealth can reduce challenges related to accessing care, while interventions involving self-management education and/or health qigong have the potential to improve COPD care and outcomes.
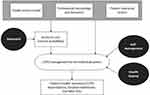 |
Figure 1 Explanatory model of causal factors influencing COPD management and outcomes, and potential approaches to enhance outcomes. |
While COPD is largely preventable, this literature underscores its persistently high prevalence and severe repercussions for individuals, healthcare systems, and economies. The lessons gleaned from the pandemic, which imposed limitations on patient access to care, resulted in suboptimal COPD management, manifesting in a decline in quality of life (QOL), heightened incidence of acute exacerbations, hospital readmissions, and elevated mortality rates.
Accumulated evidence underscores the intricacy of managing COPD and its associated comorbidities in a chronic care context. The literature review pinpointed various obstacles, spanning from individual-level factors to healthcare professionals’ knowledge, skills, and patient relationships, as well as encompassing healthcare models and their accessibility.
Based on the evidence, interventions of COPD care should address a multifaceted, innovative delivery format, supporting rural patients attending training,26,34 addressing personal and health care system environment barriers in access to care29,35 and patient and caregiver values.37
Three principal strategies emerged as efficacious means to alleviate these barriers. The first approach involves self-management education programs. Self-management education programs are safe and unlikely to cause harm.38 Several meta-analyses and systematic reviews recommended a training intensity and duration for telehealth self-management programs and supervised health qigong training to achieve significant health-improvement effects. Effective interventions are expected to improve patient’s quality of life and reduce the frequency of hospital readmission and risk of mortality.31,40,53,54,56,58,60
The telehealth delivery format can enhance access to COPD care and address the limitations of the existing health service model. The format mitigates access to care issues and enables a broader outreach for the delivery of self-management programs in patients’ homes.62 In addition, ongoing support, communication, and feedback between health professionals and participants using productive information technology via social media can enhance the professionals’ knowledge and awareness and activate patients in managing their health and wellness. It provides clinical effectiveness similar to traditional face-to-face intervention.63
Regular practice of health qigong was found to promote the health status of patients with COPD.53 It enhanced lung function,53–55,58–60 minimized the perceived severity of dyspnea,58,59 and promoted physical fitness.51,53–55,58–60 The improved physical and psychosocial health further uplifted immunity81,82 and quality of life.51,53–55,58,59
Conclusions
In conclusion, addressing the multifaceted challenges posed by COPD is imperative for optimizing patient outcomes and reducing the burden on healthcare systems worldwide. This comprehensive review underscores the critical role of personalized care in mitigating barriers related to patient factors, professional awareness, relationships, and healthcare service models. Introducing innovative approaches, such as Self-Management Educational Programs, Health Qigong, and Telehealth, presents a transformative shift towards more effective COPD management. These interventions can empower patients and enhance their physical and psychosocial well-being. By integrating these evidence-based strategies into holistic COPD management plans, healthcare providers can significantly enhance the overall quality of life for individuals grappling with this chronic condition. This collaborative effort towards comprehensive care marks a significant step forward in the global battle against COPD. Occupational therapists can effectively lead these interventions for better health and functional outcomes.
Acknowledgment
This paper is based on the dissertation of Damian Chi Hong Siu.21 It has been published on the institutional website: https://open.bu.edu/handle/2144/46172.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. World Health Statistics 2022: Monitoring Health for the Sdgs, Sustainable Development Goals. World Health Organization; 2022.
2. Vanfleteren LEGW, Spruit MA, Wouters EFM, Franssen FME. Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med. 2016;4(11):911–924. doi:10.1016/S2213-2600(16)00097-7
3. Global Initiative for Chronic Obstructive Lung Disease. 2023 global strategy for prevention, diagnosis and management of COPD. Available from: https://goldcopd.org/wp-content/uploads/2023/01/GOLD-2023-ver-1.2-7Jan2023_WMV.pdf.
4. Chan FWK, Wong FYY, Yam CHK, et al. Risk factors of hospitalization and readmission of patients with COPD in Hong Kong population: analysis of hospital admission records. BMC Health Serv Res. 2011;11(1):186. doi:10.1186/1472-6963-11-186
5. Cakir B, Gammon G. Evaluating readmission rates: how can we improve? South Med J. 2011;103(11):1079–1083. doi:10.1097/SMJ.0b013e3181f20a0f
6. Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219–1226. doi:10.1378/chest.14-2181
7. Strassels SA, Smith DH, Sullivan SD, Mahajan PS. The costs of treating COPD in the United States. Chest. 2001;119(2):344–352. doi:10.1378/chest.119.2.344
8. Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117(2):5S–9S. doi:10.1378/chest.117.2_suppl.5s
9. Gutiérrez Villegas C, Paz-Zulueta M, Herrero-Montes M, Parás-Bravo P, Madrazo Pérez M. Cost analysis of chronic obstructive pulmonary disease (COPD): a systematic review. Health Econ Rev. 2021;11(1):1–12. doi:10.1186/s13561-021-00329-9
10. Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831–838. doi:10.1136/thx.2007.086041
11. Hansel NN, McCormack MC, Kim V. The effects of air pollution and temperature on COPD. J Chron Obstruct Pulmon Dis. 2016;13(3):372–379. doi:10.3109/15412555.2015.1089846
12. Nicholson A Practicing good hygiene: why do COPD patients need to practice good hygiene? Available from: https://copd.net/pulmonary-rehab/lifestyle/practicing-good-hygiene.
13. Whittaker H, Rubino A, Müllerová H, et al. Frequency and severity of exacerbations of COPD associated with future risk of exacerbations and mortality: a UK routine health care data study. Int J Chronic Obstr. 2022;17:427–437. doi:10.2147/COPD.S346591
14. Martinez-Garcia MA, Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity? Int J Chron Obstruct Pulmon Dis. 2017;12:1401–1411. doi:10.2147/COPD.S132961
15. Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15(5):e0233147. doi:10.1371/journal.pone.0233147
16. Axson EL, Ragutheeswaran K, Sundaram V, et al. Hospitalisation and mortality in patients with comorbid COPD and heart failure: a systematic review and meta-analysis. Respir Res. 2020;21(1):54. doi:10.1186/s12931-020-1312-7
17. Njoku CM, Alqahtani JS, Wimmer BC, et al. Risk factors and associated outcomes of hospital readmission in COPD: a systematic review. Respir Med. 2020;173:105988. doi:10.1016/j.rmed.2020.105988
18. Jardim JR, Nascimento OA. The importance of inhaler adherence to prevent COPD exacerbations. Med Sci. 2019;7(4):54. doi:10.3390/medsci7040054
19. Leving MT, Kocks J, Bosnic-Anticevich S, Dekhuijzen R, Usmani OS. Relationship between peak inspiratory flow and patient and disease characteristics in individuals with COPD—A Systematic Scoping Review. Biomedicines. 2022;10(2):458. doi:10.3390/biomedicines10020458
20. Poot CC, Meijer E, Kruis AL, Smidt N, Chavannes NH, Honkoop PJ Integrated disease management interventions for patients with chronic obstructive pulmonary disease Cochrane Database Syst Rev. 2021;2021(9):
21. Siu CHD Healthy living with COPD: a telehealth self-management program for patients with chronic obstructive pulmonary disease (COPD) [dissertation on the internet] Available from: https://open.bu.edu/handle/2144/46172 (Boston, USA: Boston University); 2023.
22. Aromataris E, Fernandez RS, Godfrey C, Holly C, Khalil H, Tungpunkom P. Methodology for JBI umbrella reviews. In: Joanna Briggs Institute Reviewers’ Manual: 2014 Edition. Australia: The Joanna Briggs Institute; 2014:1–34.
23. Bhattarai B, Walpola R, Mey A, Anoopkumar-Dukie S, Khan S. Barriers and strategies for improving medication adherence among people living with COPD: a systematic review. Respir Care. 2020;65(11):1738–1750. doi:10.4187/respcare.07355
24. Bender B. Nonadherence in chronic obstructive pulmonary disease patients: what do we know and what should we do next? Curr Opin Pulm Med. 2014;20(2):132–137. doi:10.1097/MCP.0000000000000027
25. Blackstock FC, ZuWallack R, Nici L, Lareau SC. Why don’t our patients with chronic obstructive pulmonary disease listen to us? The enigma of nonadherence. Ann Am Thoracic Soc. 2016;13(3):317–323. doi:10.1513/AnnalsATS.201509-600PS
26. Cooke CE, Sidel M, Belletti DA, Fuhlbrigge AL. Review: clinical inertia in the management of chronic obstructive pulmonary disease. J Chron Obstruct Pulmon Dis. 2012;9(1):73–80. doi:10.3109/15412555.2011.631957
27. Dekhuijzen R, Lavorini F, Usmani OS, van Boven JFM. Addressing the impact and unmet needs of nonadherence in asthma and chronic obstructive pulmonary disease: where do we go from here? The Journal of Allergy and Clinical Immunology in Practice. 2018;6(3):785–793. doi:10.1016/j.jaip.2017.11.027
28. Nakken N, Janssen DJ, Bogaart EH, et al. Informal caregivers of patients with COPD: home sweet home? Eur Respir Rev. 2015;24(137):498–504. doi:10.1183/16000617.00010114
29. Cox NS, Cox NS, Dal Corso S, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2021;2021(1):CD013040. doi:10.1002/14651858.CD013040.pub2
30. Janjua S, Janjua S, Banchoff E, et al. Digital interventions for the management of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2021;2021(4):CD013246. doi:10.1002/14651858.CD013246.pub2
31. Janjua S, Janjua S, Carter D, Threapleton CJ, Prigmore S, Disler RT. Telehealth interventions: remote monitoring and consultations for people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2021;2021(7):CD013196. doi:10.1002/14651858.CD013196.pub2
32. Roberts NJ, Kidd L, Kirkwood K, Cross J, Partridge MR. A systematic review of the content and delivery of education in pulmonary rehabilitation programmes. Respir Med. 2018;145:161–181. doi:10.1016/j.rmed.2018.11.002
33. Lemmens KMM, Lemmens LC, Boom JHC, et al. Chronic care management for patients with COPD: a critical review of available evidence. J Eval Clin Pract. 2013;19(5):734–752. doi:10.1111/j.1365-2753.2011.01805.x
34. Brundisini F, Giacomini M, DeJean D, Vanstone M, Winsor S, Smith A. Chronic disease patients’ experiences with accessing health care in rural and remote areas: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser. 2013;13(15):1–33.
35. Clari M, Ivziku D, Casciaro R, Matarese M. The unmet needs of people with chronic obstructive pulmonary disease: a systematic review of qualitative findings. J Chron Obstruct Pulmon Dis. 2018;15(1):79–88. doi:10.1080/15412555.2017.1417373
36. Cox NS, Oliveria CC, Lahham A, Holland AE. Pulmonary rehabilitation referral and participation are commonly influenced by environment, knowledge, and beliefs about consequences: a systematic review using the Theore. J Physiother. 2017;63(2):84–93. doi:10.1016/j.jphys.2017.02.002
37. May CR, Cummings A, Myall M, et al. Experiences of long-term life-limiting conditions among patients and carers: what can we learn from a meta-review of systematic reviews of qualitative studies of chronic heart failure, chronic obstructive pulmonary disease and chronic kidney disease? BMJ open. 2016;6(10). doi:10.1136/bmjopen-2016-011694
38. Schrijver J, Schrijver J, Lenferink A, et al. Self‐management interventions for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2022;2023(3):CD002990. doi:10.1002/14651858.CD002990.pub4
39. Helvaci A, Gok Metin Z. The effects of nurse‐driven self‐management programs on chronic obstructive pulmonary disease: a systematic review and meta‐analysis. Journal of Advanced Nursing. 2020;76(11):2849–2871. doi:10.1111/jan.14505
40. Jolly K, Sidhu MS, Bates E, et al. Systematic review of the effectiveness of community-based self-management interventions among primary care COPD patients. NPJ Primary Care Respiratory Medicine. 2018;28(1):44–48. doi:10.1038/s41533-018-0111-9
41. Lenferink A, Brusse-Keizer M, van der Valk Paul M, et al. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;2017(8):CD011682. doi:10.1002/14651858.CD011682.pub2
42. Newham JJ, Presseau J, Heslop-Marshall K, et al. Features of self-management interventions for people with COPD associated with improved health-related quality of life and reduced emergency department visits: a systematic review and meta-analysis. Int J Chronic Obstr. 2017;12:1705–1720. doi:10.2147/COPD.S133317
43. O’Connell S, McCarthy VJ, Savage E. Self-management support preferences of people with asthma or chronic obstructive pulmonary disease: a systematic review and meta-synthesis of qualitative studies. Chro Illn. 2021;17(3):283–305 doi:10.1177/1742395319869443.
44. Song X, Hallensleben C, Zhang W, et al. Blended self-management interventions to reduce disease burden in patients with chronic obstructive pulmonary disease and asthma: systematic review and meta-analysis. J Med Int Res. 2021;23(3):e24602. doi:10.2196/24602
45. Wang T, Tan J, Xiao LD, Deng R. Effectiveness of disease-specific self-management education on health outcomes in patients with chronic obstructive pulmonary disease: an updated systematic review and meta-analysis. Patient Educ Couns. 2017;100(8):1432–1446. doi:10.1016/j.pec.2017.02.026
46. Shnaigat M, Downie S, Hosseinzadeh H. Effectiveness of health literacy interventions on COPD Self-management outcomes in outpatient settings: a systematic review. J Chron Obstruct Pulmon Dis. 2021;18(3):367–373. doi:10.1080/15412555.2021.1872061
47. Barrecheguren M, Bourbeau J. Self-management strategies in chronic obstructive pulmonary disease: a first step toward personalized medicine. Curr Opin Pulm Med. 2018;24(2):191–198. doi:10.1097/MCP.0000000000000460
48. Bourbeau J, Echevarria C. Models of care across the continuum of exacerbations for patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2020;17:1479973119895457 doi:10.1177/1479973119895457.
49. Cravo A, Attar D, Freeman D, Holmes S, Ip L, Singh SJ. The importance of self-management in the context of personalized care in COPD. Int J Chronic Obstr. 2022;17:231–243. doi:10.2147/COPD.S343108
50. Nohra R, Serhal R, Sacre H, Salameh P, Rothan-Tondeur M. Effective components of self-management programs for chronic obstructive pulmonary disease patients: scoping review. Adv Respir Med. 2020;88(3):223–232. doi:10.5603/ARM.2020.0117
51. Guo J, Chen B, Lu Y, et al. Tai Chi for improving cardiopulmonary function and quality of life in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Clin rehabilitat. 2016;30(8):750–764. doi:10.1177/0269215515604903
52. Cao A, Feng F, Zhang L, Zhou X. Baduanjin exercise for chronic obstructive pulmonary disease: an updated systematic review and meta-analysis. Clin rehabilitat. 2020;34(8):1004–1013. doi:10.1177/0269215520926635
53. Guo C, Xiang G, Xie L, et al. Effects of Tai Chi training on the physical and mental health status in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Thoracic Dis. 2020;12(3):504–521. doi:10.21037/jtd.2020.01.03
54. Liu X, Fu C, Hu W, et al. The effect of Tai Chi on the pulmonary rehabilitation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(4):3763–3782. doi:10.21037/apm-20-940
55. Tong H, Liu Y, Zhu Y, Zhang B, Hu J. The therapeutic effects of qigong in patients with chronic obstructive pulmonary disease in the stable stage: a meta-analysis. BMC Complementary and Alternative Medicine. 2019;19(1):239. doi:10.1186/s12906-019-2639-9
56. Cai Q, Cai S, Chen J, et al. Tai Chi for anxiety and depression symptoms in cancer, stroke, heart failure, and chronic obstructive pulmonary disease: a systematic review and meta-analysis. Complementary Therapies in Clinical Practice. 2022;46:101510. doi:10.1016/j.ctcp.2021.101510
57. Li Z, Liu S, Wang L, Smith L. Mind–body exercise for anxiety and depression in COPD patients: a systematic review and meta-analysis. Int J Environ Res Public Health. 2019;17(1):22. doi:10.3390/ijerph17010022
58. Ngai SP, Jones AY, Tam WWS, Ngai SP. Tai Chi for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2016;2016(6):CD009953. doi:10.1002/14651858.CD009953.pub2
59. Zhang H, Hu D, Xu Y, Wu L, Lou L. Effect of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized controlled trials. Ann Med. 2022;54(1):262–273. doi:10.1080/07853890.2021.1999494
60. Reychler G, Poncin W, Montigny S, Luts A, Caty G, Pieters T. Efficacy of yoga, tai chi and qi gong on the main symptoms of chronic obstructive pulmonary disease: a systematic review. Respirat Med Res. 2019;75:13–25. doi:10.1016/j.resmer.2019.04.002
61. Bonnevie T, Smondack P, Elkins M, et al. Advanced telehealth technology improves home-based exercise therapy for people with stable chronic obstructive pulmonary disease: a systematic review. J Physiother. 2021;67(1):27–40. doi:10.1016/j.jphys.2020.12.006
62. McCabe C, McCann M, Brady AM, McCabe C. Computer and mobile technology interventions for self‐management in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;5(5):CD011425. doi:10.1002/14651858.CD011425.pub2
63. Michaelchuk W, Oliveira A, Marzolini S, et al. Design and delivery of home-based telehealth pulmonary rehabilitation programs in COPD: a systematic review and meta-analysis. Int J Med Inform. 2022;162:104754. doi:10.1016/j.ijmedinf.2022.104754
64. Shaw G, Whelan ME, Armitage LC, Roberts N, Farmer AJ. Are COPD self-management mobile applications effective? A systematic review and meta-analysis. NPJ Primary Care Respiratory Medicine. 2020;30(1):11. doi:10.1038/s41533-020-0167-1
65. Selzler A, Wald J, Sedeno M, et al. Telehealth pulmonary rehabilitation: a review of the literature and an example of a nationwide initiative to improve the accessibility of pulmonary rehabilitation. Chron Respir Dis. 2018;15(1):41–47 doi:10.1177/1479972317724570.
66. Tashkin DP. Multiple dose regimens. Impact on compliance. Chest. 1995;107(5 Suppl):176S–182S. doi:10.1378/chest.107.5_Supplement.176S
67. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi:10.1097/01.mlr.0000114908.90348.f9
68. Phillips LS, Branch WT, Cook CB, et al. Clinical Inertia. Ann Internal Med. 2001;135(9):825–834. doi:10.7326/0003-4819-135-9-200111060-00012
69. Allen JD, Curtiss FR, Fairman KA. Nonadherence, Clinical Inertia, or Therapeutic Inertia? J Managed Care Pharm. 2009;15(8):690–695. doi:10.18553/jmcp.2009.15.8.690
70. Barton C, Effing TW, Cafarella P. Social support and social networks in COPD: a scoping review. J Chron Obstruct Pulmon Dis. 2015;12(6):690–702. doi:10.3109/15412555.2015.1008691
71. Lowe KE, Make BJ, Crapo JD, et al. Association of low income with pulmonary disease progression in smokers with and without chronic obstructive pulmonary disease. ERJ Open Res. 2018;4(4):69. doi:10.1183/23120541.00069-2018
72. Johns DP, Walters JAE, Walters EH. Diagnosis and early detection of COPD using spirometry. J Thoracic Dis. 2014;6(11):1557–1569. doi:10.3978/j.issn.2072-1439.2014.08.18
73. Marques A, Cruz J, Brooks D. Interventions to support informal caregivers of people with chronic obstructive pulmonary disease: a systematic literature review. Respiration. 2021;100(12):1230–1242. doi:10.1159/000517032
74. Davis KJ, Landis SH, Oh Y, et al. Continuing to Confront COPD International Physician Survey: physician knowledge and application of COPD management guidelines in 12 countries. Int J COPD. 2015;10:39–55. doi:10.2147/COPD.S70162
75. Han M, Fu Y, Ji Q, Deng X, Fang X. the effectiveness of theory-based smoking cessation interventions in patients with chronic obstructive pulmonary disease: a meta-analysis. BMC Public Health. 2023;23(1):1510. doi:10.1186/s12889-023-16441-w
76. Menezes AM, Landis SH, Han MK, et al. Continuing to Confront COPD International Surveys: comparison of patient and physician perceptions about COPD risk and management. Int J Chronic Obstr. 2015;10(1):159–172. doi:10.2147/COPD.S74315
77. Ogunbayo OJ, Russell S, Newham JJ, et al. Understanding the factors affecting self-management of COPD from the perspectives of healthcare practitioners: a qualitative study. NPJ Primary Care Respiratory Medicine. 2017;27(1):54–59. doi:10.1038/s41533-017-0054-6
78. Park HJ, Byun MK, Kim T, et al. Frequent outpatient visits prevent exacerbation of chronic obstructive pulmonary disease. Sci Rep. 2020;10(1):6049. doi:10.1038/s41598-020-63064-x
79. Bourbeau J, Farias R. Making sense of telemedicine in the management of COPD. Europ resp J. 2018;51(5):1800851. doi:10.1183/13993003.00851-2018
80. Forge L, Ralph MS. Aligning mind and body: exploring the disciplines of mindful exercise. ACSMs Health Fit J. 2005;9(5):7–14.
81. Xiao C, Zhuang Y. Efficacy of liuzijue qigong in individuals with chronic obstructive pulmonary disease in remission. J Am Geriatr Soc. 2015;63(7):1420–1425. doi:10.1111/jgs.13478
82. Tsang HWH, Jones AYM, So CT, Mok TYW. Functional and psychosocial effects of health qigong in patients with COPD: a randomized controlled trial. J Altern Complementary Med. 2011;17(3):243–251. doi:10.1089/acm.2010.0215
83. Sculley JA, Musick H, Krishnan JA. Telehealth in chronic obstructive pulmonary disease: before, during, and after the coronavirus disease 2019 pandemic. Curr Opin Pulm Med. 2022;28(2):93–98. doi:10.1097/MCP.0000000000000851
84. Alghamdi SM, Rajah AMA, Aldabayan YS, Aldhahir AM, Alqahtani JS, Alzahrani AA. Chronic obstructive pulmonary disease patients’ acceptance in E-health clinical trials. Int J Environ Res Public Health. 2021;18(10):5230. doi:10.3390/ijerph18105230
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
