Back to Journals » Journal of Blood Medicine » Volume 15
Cardiovascular Consequences of Sickle Cell Disease
Authors Bahashwan S, Almuhanna RM, Al Hazza MT , Baarma RW , AlNajjar AY, Siddiqui FS, Fatani SZ, Barefah A, Alahwal H , Almohammadi A, Radhwi O , Algazzar AS, Mansory EM
Received 18 January 2024
Accepted for publication 27 April 2024
Published 6 May 2024 Volume 2024:15 Pages 207—216
DOI https://doi.org/10.2147/JBM.S455564
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Salem Bahashwan,1,2 Rahaf Mohammad Almuhanna,3 Maryam Taher Al Hazza,1 Reem Wajdi Baarma,1 Abdulrahman Yousif AlNajjar,4 Faris Sameer Siddiqui,1 Shouq Ziyad Fatani,5 Ahmed Barefah,1,2 Hatem Alahwal,1,2 Abdullah Almohammadi,1,2 Osman Radhwi,1,2 Alaa S Algazzar,6 Eman M Mansory1,2
1Hematology Department, Faculty of Medicine, King Abdulaziz University, Jeddah, 21589, Saudi Arabia; 2Hematology Research Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, 21589, Saudi Arabia; 3Emergency Medicine Department, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia; 4Emergency Medicine Department, King Abdullah Medical Complex, Jeddah, Saudi Arab; 5Physical Medicine and Rehabilitation, Prince Sultan Military Medical City, Riyadh, Saudi Arabia; 6Cardiology Department,Ahmed Maher Teaching hospital, Cairo, Egypt
Correspondence: Salem Bahashwan, Hematology Department, Faculty of Medicine, King Abdulaziz University, Jeddah, 21589, Saudi Arabia, Tel +966555511968, Email [email protected]
Background: Sickle cell disease is an inherited blood disorder which can lead to severe complications, particularly in the cardiovascular and respiratory systems, potentially resulting in arrhythmias, pulmonary hypertension (PH), and cardiomegaly. This study aims to investigate the risk of PH and arrhythmias in adult SCD patients.
Methods: Retrospective analysis of medical records from King Abdulaziz University Hospital (KAUH) for patients with SCD aged 15 and above between 2009 and 2021. The study included 517 patients, with echocardiograms and electrocardiograms assessed according to the European Society of Cardiology/the European Respiratory Society (ESC/ERS) guidelines for categorizing PH risk (low, moderate, high) and detecting arrhythmias. Data analysis employed the Statistical Package for the Social Sciences (SPSS), utilizing quantitative and qualitative data representation. Multivariate logistic regression identified independent risk factors with odds ratios at a 95% confidence interval (CI).
Results: Among participants, 50.3% were male, with a total sample average age of 34.45 ± 9.28 years. Results indicated that 1.4% of patients experienced arrhythmias, 3.7% had a moderate PH risk, and 3.3% were classified as high PH risk. Logistic regression revealed significant independent risk factors for PH and arrhythmia in patients with SCD, with chronic kidney disease (CKD) carrying the highest odds (26.4 times higher odds of PH and 15.36 times higher odds of arrhythmias).
Conclusion: Patients with SCD are at risk for developing PH and various arrhythmias but are often underdiagnosed. Key risk factors for PH included CKD, liver cirrhosis, and pre-existing cardiac conditions. Arrhythmias were significantly associated with CKD and pre-existing cardiac conditions. To mitigate these risks, we recommend involving a multidisciplinary healthcare team in the care of adult patients with SCD. Future prospective studies are advised for early detection of PH and arrhythmias in hemoglobinopathy patients, potentially reducing mortality.
Keywords: sickle cell disease, arrhythmia, pulmonary hypertension, chronic kidney disease, hemoglobinopathies, PH risk
Graphical Abstract:
Introduction
Sickle cell disease (SCD) is a group of genetic red blood cell disorders, inherited as an autosomal recessive condition characterized by the presence of an abnormal hemoglobin S leading to polymerization of hemoglobin in the deoxygenated state causing changes and distortion in the shape of red blood cells and reduction of the flexibility of the hemoglobin.1–4 Sickle cell disease has two major components participating and playing a major role in most of the complications related to SCD: vaso-occlusive crises and hemolysis.2,5 During the inflammatory state, the interaction that happens between these distorted red blood cells with the white blood cells and the endothelium will lead to adhesion of this complex and will result in the occlusion of the blood vessels causing ischemia to different vital organs, including blood vessels supplying the cardio-respiratory and the cardio-vascular systems.6 Sickle cell disease in general involves many other subgroups under its umbrella, including sickle cell anemia, where the only abnormality is the presence of hemoglobin S; hemoglobin SC disease, where the hemoglobin S is presented with another mutation in the beta-globin chain of the hemoglobin forming hemoglobin C; and hemoglobin sickle beta thalassemia, as the patient is carrying both mutation of hemoglobin S and the mutation of beta thalassemia at the same time.5 Since the first description of SCD more than a hundred years ago, our understanding of this inherited disease has dramatically improved and tis reflected in a positive way in patient care and management5,7,8 This evolution started in 1984 with the first reported utilization of hydroxyurea in SCD patients to increase the level of fetal hemoglobin and decrease the crises and complications related to the disease9 This evolution has continued in the last 10 years by introducing new agents such as voxelotor, crizanlizumab, L-glutamin,10,11 and, most recently, gene therapy12 The adoption of some guidelines and methods in the care and management of SCD, such as vaccination and intensive screening, to address different expected complications has significantly decreased morbidity and mortality.1,13 A big difference is now noticed in morbidity and mortality between high income and low income countries, as training of healthcare providers and access to services are much more easily provided in high income countries. These disorders may also cause fatal complications, especially cardiopulmonary issues such as arrhythmias, tachycardia, pulmonary hypertension (PH), and cardiomegaly. Pulmonary hypertension is a pathophysiological condition with variable clinical presentations in the early and late stages of multiple clinical conditions, which can affect directly or indirectly the respiratory and cardiovascular systems.3,14 It is defined as a resting mean pulmonary artery pressure (mPAP) of ≥25 mmHg, with a mean pulmonary artery wedge pressure (PAWP) or left ventricular end diastolic pressure (LVEDP) of ≤15 mm Hg plus increased pulmonary vascular resistance (PVR).3 PH is of great importance and should be viewed with special consideration in patients with SCD as it is relatively common in those patients, and has significant implications for morbidity and mortality8 The early symptoms of pulmonary hypertension in sickle cell patients are usually non-specific and do not differ from symptoms presented in SCD with no pulmonary hypertension.14,15 Pulmonary hypertension is usually confirmed by right heart catheterization, but there are some limitation of using this method in all suspected cases of pulmonary hypertension, as it is too expensive, is an invasive procedure, and is not available and accessible everywhere. Other non-invasive methods such as Doppler echo and NT-pro-BNP, which are not accurate enough to diagnose pulmonary hypertension and do not replace right side catheterization, are acceptable as alternative methods to identify and screen the group of patients at high risk of developing pulmonary hypertension.16 Pulmonary hypertension is classified into five general groups: pulmonary arterial hypertension, pulmonary hypertension due to left heart disease, pulmonary hypertension due to lung disease and / or hypoxia, chronic thrombo-embolic pulmonary hypertension, and pulmonary hypertension with unclear or multifactorial mechanisms. Pulmonary hypertension related to SCD and hemolysis was initially classified under the category of pulmonary arterial hypertension in the published guidelines in 2009, which was written by the task force for treatment and diagnosis of pulmonary hypertension, a collaborative work between the European Respiratory Society and the European Society of Cardiology.17 The classification of pulmonary hypertension associated with SCD and hemolysis was then changed in 2013 at the Fifth World Symposium on Pulmonary Hypertension, and is now classified under the category of pulmonary hypertension with unclear or multiple etiology.18 Depending on the hemodynamics of the cardio-respiratory circulation, pulmonary hypertension can be subdivided into two major categories: precapillary and post-capillary pulmonary hypertension. The subtype is usually recognized and categorized during the procedure of right heart catheterization locoing for and calculating two variables: pulmonary capillary wedge pressure and left ventricular end diastolic pressure. If one of these two variables is greater than 15, pulmonary hypertension will be categorized as post-capillary pulmonary hypertension; if one of the two variables is less than or equal to 15, it will be considered to be precapillary pulmonary hypertension. In SCD patients, there is an equal distribution between precapillary and post-capillary pulmonary hypertension among those who have pulmonary hypertension.19 Several risk factors were identified to contribute to the pathogenesis of PH in patients with SCD, including ongoing hemolysis and hypoxic pulmonary vasoconstriction, microvascular occlusion, left ventricle dysfunction as well as a chronic inflammatory state, decreased nitrous oxide and hypercoagulability. In SCD patients, any oxidatively stressed environment can lead to the production of a reactive oxygen species (ROS), which can damage the endothelium; this ROS is found to be inhibited by nitrous oxide synthase. A decreased level of nitric oxide can enhance red blood cell adhesion to the endothelium.20,21 With regard to cardiac arrhythmias, it is defined as irregularities in the rhythm of the heartbeat, which could be either bradycardia or tachycardia; it affects all age groups. With recent advances in understanding the underlying electrophysiology of the heart and the development of arrhythmias, two major mechanisms of arrhythmias have been identified: enhanced or abnormal impulse formation or conduction disturbances.22
A study conducted in the United States concluded that the prevalence of PH among patients with SCD was approximately 30%, subcategorized into 17% mild, 8% moderate, and 3% severe. The prevalence was noticed to be higher in the group of SCD patients who had higher fetal hemoglobin level and lower systolic blood pressure.23 This is in keeping with another single-center study, conducted in Saudi Arabia, where prevalence was found to be 38%, with most being sub-categorized as mild PH; higher prevalence was noticed in the group of SCD patients who had a higher serum ferritin level and a lower fetal hemoglobin level.24 Studies that performed right heart catheterization for patients with elevated peak tricuspid regurgitant jet velocity reported a confirmed diagnosis of PH in 10% of patients with SCD. Higher prevalence was demonstrated in those patients with SCD who had a lower hematocrit level, and higher levels of direct bilirubin, aspartate aminotransferase, lactate dehydrogenase, and serum ferritin.25
Accumulating evidence suggests that the diagnosis of PH in patients with SCD acts as an independent predictor of mortality.14 One study based on an estimate of median survival time of about 6.8 years after the diagnosis of PH found that those those with pulmonary hypertension died at a younger age compared to those without.25
As for arrhythmias, a study in the United States concluded that paroxysmal atrial fibrillation was the most common arrhythmia among patients with SCD crises, followed by supraventricular tachycardia, long QT syndrome, atrial flutter, and ventricular fibrillation.26 In addition, a study among inpatients with SCD in the United States showed that 3.4% of them had documented arrhythmias. Of SCD-related admissions, 60% were associated with arrhythmia, with an obvious and clear rise in prevalence in subsequent years that significantly impacted care as it was associated with higher mortality in SCD patients and increased duration of hospitalization.27
After reviewing the literature, there were no updated studies regarding the incidence of PH risk and arrhythmias in adult patients with SCD in Saudi Arabia. Thus, we aimed to analyze it and provide data for future research, retrospectively.
Subjects and Methods
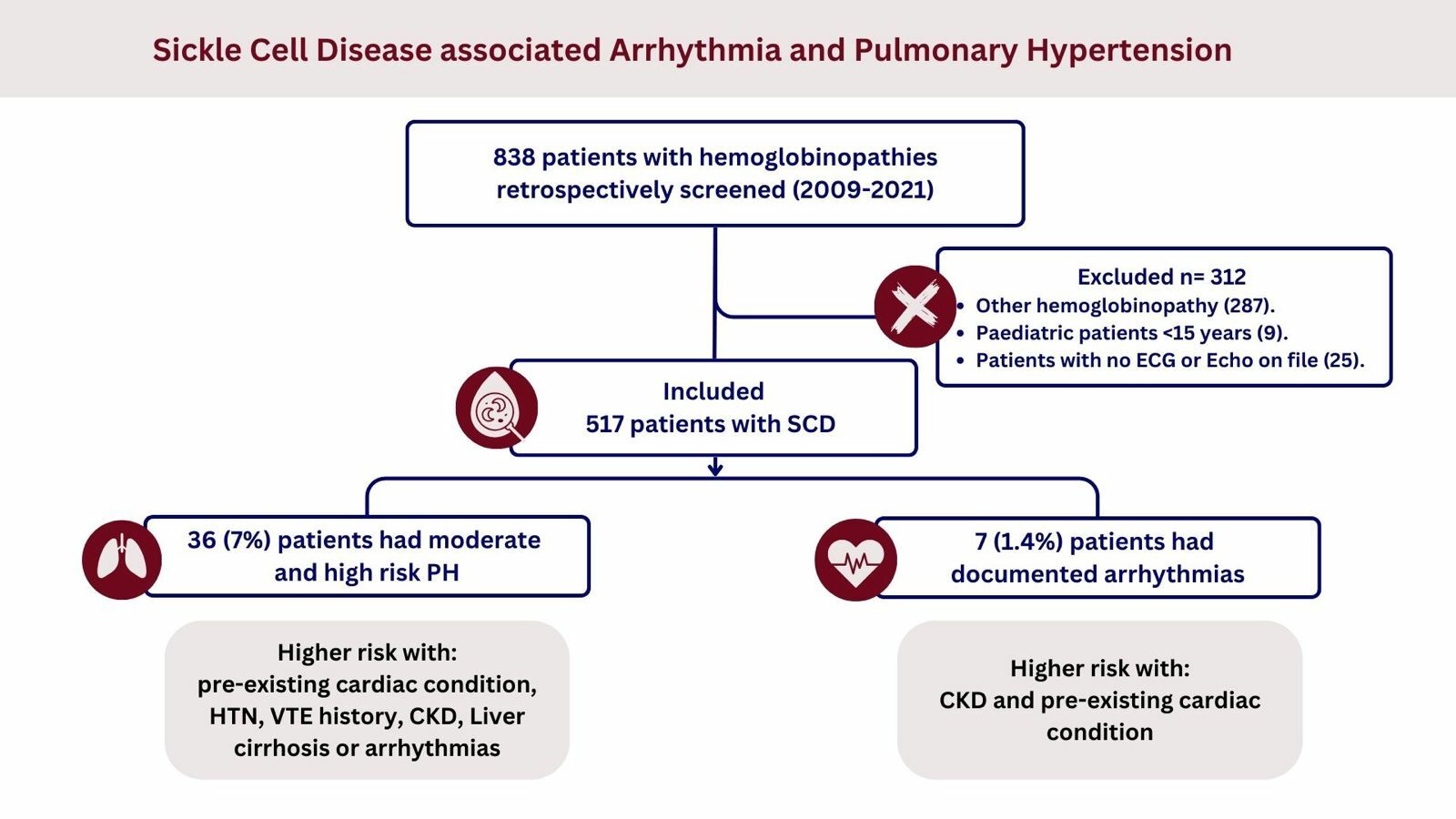
This retrospective study was done using hospital medical records at King Abdulaziz University Hospital (KAUH), a major tertiary care center in Jeddah, Saudi Arabia. We obtained ethical approval from the Unit of Biomedical Ethics Research Committee of KAUH (reference number 565–21; approved on 28 November 2023). Informed consent was not required and was waived by the ethical committee as this study was a retrospective non-interventional study, and all information was collected from the medical records in the hospital. The data were anonymized and maintained with confidentiality in the office of the corresponding author in compliance with the Declaration of Helsinki. This study included all patients with a diagnosis of sickle cell disease who were followed between 2009 and 2021. We excluded patients with sickle cell anemia trait, pediatric patients below 15 years, and those who had never undergone an echocardiogram or electrocardiogram (ECG). Initially, 838 patients were screened, and after the application of the exclusion criteria, 517 patients’ medical records were reviewed. All included patients had their records examined by a consultant cardiologist to screen for arrhythmias and assess the risk for PH according to the tricuspid regurgitation velocity observed in their echo, as adapted from the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines.28 Thereafter the patients were categorized into three risk groups: high risk when peak tricuspid regurgitation velocity (TVR) is 2.9−3.4 with other PH signs on echo or when >3.4; moderate risk when peak TVR is ≤2.8 or not measurable in the presence of other echo PH signs or peak TVR is 2.9–3.4 with no other PH signs; and low risk when peak TRV is ≤2.8 or not measurable, with no other echo PH signs.
For the purpose of data collection, a pre-designed checklist was prepared to collect data about patients’ demographics (age, gender), death, type of hemoglobinopathy, comorbidities (cardiovascular diseases, diabetes mellitus, thyroid disease, history of pulmonary embolism (PE) or deep vein thrombosis (DVT), chronic kidney disease (CKD), pre-existing cardiac conditions (this included ischemic heart disease, valvular heart disease or rheumatic heart disease, liver cirrhosis, and hypertension [HTN]), in addition to patients’ echocardiography reports and ECGs.
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) program, version 26. To test the relationship between variables, qualitative data was expressed as numbers and percentages, and the chi-squared test was used to test the relationship between variables. Quantitative data was expressed as mean and standard deviation (mean ± SD). Multivariate logistic regression analysis was done to assess the association of risk factors with developing arrhythmias among studied patients. In addition, a multivariate ordered logistic regression model assessing the association of risk factors and risk for pulmonary hypertension was done. The odds ratio was calculated, and 95% confidence intervals (CIs) were reported. A p-value of less than 0.05 was considered statistically significant.
Results
A total of 517 patients diagnosed with SCD were reviewed. Of these patients, 257 (49.7%) were female and 260 (50.3%) were male. Mean age was 34.45 ± 9.28 years. Upon investigation of different comorbidities, pre-existing cardiac conditions were the most prevalent (10.6%). Moreover, 7 (1.4%) had an arrhythmic episode, and 19 (3.7%) had a moderate risk of pulmonary hypertension and 17 (3.3%) were considered high risk. Of the included sample, 46 (8.9%) patients were deceased, with a mean age at death of 33 ± 10.3. Among those patients who had an arrhythmic episode, 2 developed atrial fibrillation, 3 developed first-degree heart block, 1 had supra-ventricular tachycardia, and 1 had a pulseless electrical activity. Demographic details and percentage distributions among studied patients are shown in Table 1. Based on echocardiography assessment, 11 (4.2%) and 10 (3.8%) males were found to have a high and moderate risk of PH, respectively, compared to 6 (2.3%) females with a high risk of PH and 9 (3.5%) with a moderate risk; there were no significant differences according to sex (p=0.467). Investigating the effect of the copresence of other comorbidities, PH showed a significant association with the following pre-existing cardiac conditions: venous thromboembolism, liver and kidney disease, and hypertension (p<0.000). In addition, among deceased patients, 7 (15.2%) had a high risk of PH while 2 (4.3%) had a moderate risk (p<0.000). Details of PH and its relationship to other variables are provided in Table 2. As for arrhythmias, most patients were male (5, 71.4%), in comparison to 2 females (28.6%), with no significant difference (p=0.26). There was a significant association between the presence of cardiac conditions and the development of arrhythmia, with 4 patients (57.1%) having pre-existing cardiac conditions (p<0.004). However, there was no significant association between having a history of PE and DVT with arrhythmias and not having such a history (1 patient [14.3%] and 6 patients [85.7%], respectively; p=0.259). Among patients with chronic kidney disease, two had a recorded incidence of arrhythmias, with a significant difference (p<0.001). Finally, there was a significant association between having PH and developing arrhythmias; two (28.6%) patients with arrhythmias were found to have a high risk of PH while three (42.9%) had a moderate risk (p<0.000) (Table 3).
 |
Table 1 Distribution of Studied Patients According to Their Demographics, Chronic Diseases, PH Risks and Outcomes (N=517) |
 |
Table 2 Relationship Between Prevalence of Pulmonary Hypertension Risk and Patients’ Demographics, Chronic Diseases, and Outcomes (N=517) |
 |
Table 3 Relationship Between Prevalence of Arrhythmias and Patients’ Demographics, Chronic Diseases, and Outcomes (N=517) |
Logistic regression analysis was done to evaluate for independent risk factors and multiple significant results were noted (Tables 4 and 5). CKD, liver cirrhosis, arrhythmias, history of PE or DVT, HTN, and pre-existing cardiac conditions were all found to be independent risk factors for pulmonary hypertension in patients with sickle cell disease. Furthermore, patients with CKD were found to have 26.4 times higher odds of developing pulmonary hypertension than their counterparts (95% CI: 2.76–253.19; p=0.005). Also, patients with arrhythmias had 8.31 times increased odds of pulmonary hypertension (95% CI: 1.29–53.67; p=0.026). On the other hand, thyroid disease was found to have no significant difference regarding pulmonary hypertension risk (p=0.146).
 |
Table 4 Multivariate Ordered Logistic Regression for Pulmonary Hypertension |
 |
Table 5 Multivariate Logistic Regression for Arrhythmias |
Additionally, patients with CKD were found to have 15.36 times higher odds of developing cardiac arrhythmias in comparison to patients without CKD (95% CI: 1.77–133.27; p=0.013); however, patients with pre-existing cardiac conditions were found to have 6.28 times higher odds than patients without them (95% CI: 1.09–35.96; p=0.039).
Discussion
Patients with sickle cell disease are at high risk for many complications, including cardiac and pulmonary complications. In this study we retrospectively reviewed SCD patients at a tertiary care center in a region with a high prevalence of the disease. Sickle cell disease patients who had undergone echocardiographic evaluation and had had an ECG assessment were analyzed. The study found that around 3.3% of patient had a high risk of PH and 3.7% had a moderate risk based on tricuspid regurgitation velocity observed in their echo, as adapted from ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension.28 Patients with other comorbidities, including CKD, liver cirrhosis, arrhythmias, VTE, HTN, and pre-existing cardiac conditions, were found to have higher odds of being at risk of PH. Only 1.4% of the patients assessed had arrythmia; a history of CKD and pre-existing cardiac conditions carried the highest odds.
Though echocardiographic assessment can suggest a diagnosis of PH and establish a risk category based on peak TVR velocity, a right heart catheterization is usually required to confirm diagnosis as it is the gold standard test.29 Studies that look at patients with SCD who have undergone right heart catheterization are likely under-reporting the prevalence of this important complication in this vulnerable patient population; the invasive nature of this procedure and the cost and technical difficulties associated with it do not allow all patients to undergo such a workup. Therefore, in this study we preferred to evaluate the risk of PH by echocardiography to capture all symptomatic and asymptomatic SCD patients.
The significant association between VTE and risk of PH reported in this study (p<0.000) is in keeping with a similar study which explained how sickle cell disease by itself is associated with chronic hypercoagulable states via various mechanisms, including the chronic hemolysis that increases the risk of VTE but also contributes to the pathogenesis behind developing PH. The researchers also found an increased risk of VTE by 30% or more depending on the SCD genotype in patients with pulmonary hypertension. The occurrence of thromboembolic events in the pulmonary blood vessels is underestimated and sometimes misdiagnosed in SCD patients, as some of them will have no signs or symptoms of acute venous thromboembolism or pulmonary embolism. However, when imaging by CT scan or ventilation perfusion scan of the lung is performed for any reason, an incidental finding of a thromboembolic event or its consequences is usually found. This was supported in a study that demonstrated the presence of acute thromboembolic events in more than 50% of SCD patients, and a ventilation perfusion scan of the lung revealed mismatched segmental perfusion defects in around 80% of cases.30 This finding signifies the need for further preventative measures to ensure reduction of VTE risk in this patient group.31 In addition, this study shows what several studies have documented before regarding the significant relation between pre-existing cardiac conditions, pulmonary hypertension, and SCD.32,33 Furthermore, pulmonary hypertension risk was associated with chronic kidney disease (p<0.000); all six patients had a moderate to high risk (33.3% and 66.7%, respectively). This finding is consistent with another study that postulated common mechanisms shared in the pathogenesis of CKD that could also exacerbate or induce PH, such as volume overload, severe anemia, and left ventricular dysfunction. However, this study was not specific to patients with sickle cell disease.34 Moreover, the study commented that correcting volume overload and treatment of left ventricular disorders can help reduce PH in patients with CKD. The statistically significant association between pulmonary hypertension and increased mortality in patients with SCD shown in this study has been documented in previous prospective and registry-based studies.35,36 This finding calls for more attention to implementing strategies to enhance early diagnosis of PH and trials to assess treatment options and to establish guidelines for the follow-up and management of SCD patients.
The anemia and polymerization of hemoglobin can lead to cardiac involvement in SCD, whether it is left or right ventricular dysfunction.37 In this study, we investigated the presence of arrhythmias in patients with SCD. Only seven patients were found to have arrhythmias, while in a similar study, which included 100 patients with SCD, 41% were found to have arrhythmias, mainly sinus tachycardia.38 The discrepancy of our finding compared to the other aforementioned study could be due to the timing of ECG assessments, which were conducted outside the occurrence of arrhythmia, and potentially influenced the observed results. Additionally, 5 out of 7 patients with arrhythmias were found to have a moderate to high risk of PH (p<0.000). Similarly, the relationship between PH and arrhythmias has been reported in previous studies; one concluded that QTc dispersion is associated with SCD, especially in those patients with PH.39 Moreover, about 50% of patients with CKD had arrhythmias with a significant difference (p<0.000). It was noted in a previous study that patients on hemodialysis have various arrhythmic triggers, including left ventricular dysfunction and fluid overload.34 Despite the low incidence of arrhythmias found in SCD patients, most of the events were observed in the sub-groups who have CKD or a high risk of developing PH, suggesting that screening for arrhythmias is recommended in those sub-groups. In addition, hemodialysis is associated with significant alterations in electrolyte levels, with gradual shifts occurring between dialysis sessions and rapid changes during the dialysis procedure. The established risk of arrhythmias related to these electrolyte fluctuations, along with the chronic and often severe electrolyte imbalances prevalent in individuals with CKD, is widely recognized.40 Therefore, implementing cardiovascular risk modification and prevention of electrolyte disturbances can be beneficial.40 In this study, arrhythmias were found to have no significant association with death as an outcome in patient condition (p=0.066), contrary to other studies in the literature that mention them as a risk factor for increased mortality and length of stay.38 Disparity in this result could be due to the small number of documented arrhythmic patients in our data, or maybe the presence of other comorbidities associated with arrhythmia in SCD patients in the other literature described here contributed to the outcome directly or indirectly.
The strength of this study mainly comes from representing real-world data in a country with a high prevalence of sickle cell disease. On the other side, this study has many limitations, mainly its retrospective nature and the challenges related to patients’ loss of follow-up, which may introduce gaps in patient data. Another limitation is that the documented cases of arrhythmia were small and we could not be certain in predicting their morbidity and mortality.
In conclusion, adult patients with SCD are at risk of developing pulmonary hypertension and various arrhythmias. However, this risk is often underestimated and underdiagnosed in this population despite their great significance and effect on patients’ survival. This study highlights the importance of strategies that can potentially serve as a preventative approach, such as: addressing fluid and electrolyte imbalances, implementing interventions to lower cardiovascular risk, and involving a multidisciplinary healthcare team in the care of adult SCD patients, even if they are asymptomatic. Moreover, further research is needed regarding the relationship between chronic kidney disease and pulmonary hypertension in the setting of sickle cell disease, which can help to improve our understanding and hopefully achieve better care of such patients. Also, studies using a prospective design are recommended for better identification of pulmonary hypertension and arrhythmias in patients with SCD to establish screening programs for early prediction and decreased mortality.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kavanagh PL, Fasipe TA, Wun T. Sickle cell disease. JAMA. 2022;328(1):57. doi:10.1001/jama.2022.10233
2. Wood KC, Gladwin MT, Straub AC. Sickle cell disease: at the crossroads of pulmonary hypertension and diastolic heart failure. Heart. 2020;106(8):562–568. doi:10.1136/heartjnl-2019-314810
3. Hayes MM, Vedamurthy A, George G, et al. Pulmonary hypertension in sickle cell disease. Ann Am Thorac Soc. 2014;11(9):1488–1489. doi:10.1513/AnnalsATS.201408-405CME
4. Steppan J, Tran HT, Bead VR, et al. Arginase inhibition reverses endothelial dysfunction, pulmonary hypertension, and vascular stiffness in transgenic sickle cell mice. Anesth Analg. 2016;123(3):652–658. doi:10.1213/ANE.0000000000001378
5. Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4(1):18010. doi:10.1038/nrdp.2018.10
6. Langer HF, Chavakis T. Leukocyte – endothelial interactions in inflammation. J Cell Mol Med. 2009;13(7):1211–1220. doi:10.1111/j.1582-4934.2009.00811.x
7. Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. doi:10.1056/NEJM199505183322001
8. Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–895. doi:10.1056/NEJMoa035477
9. Agrawal RK, Patel RK, Shah V, Nainiwal L, Trivedi B. Hydroxyurea in sickle cell disease: drug review. Indian J Hematol Blood Transfusion. 2014;30(2):91–96. doi:10.1007/s12288-013-0261-4
10. Migotsky M, Beestrum M, Badawy SM. Recent advances in sickle-cell disease therapies: a review of voxelotor, crizanlizumab, and L-glutamine. Pharmacy. 2022;10(5). doi:10.3390/pharmacy10050123
11. Leibovitch JN, Tambe AV, Cimpeanu E, et al. l-glutamine, crizanlizumab, voxelotor, and cell-based therapy for adult sickle cell disease: hype or hope? Blood Rev. 2022;53:100925. doi:10.1016/j.blre.2021.100925
12. Leonard A, Tisdale JF. Gene therapy for sickle cell disease. Hematology. 2023;2023(1):542–547. doi:10.1182/hematology.2023000487
13. Ogu UO, Badamosi NU, Camacho PE, Freire AX, Adams-Graves P. Management of sickle cell disease complications beyond acute chest syndrome. J Blood Med. 2021;12:101–114. doi:10.2147/JBM.S291394
14. Sheikh AB, Nasrullah A, Lopez ED, et al. Sickle cell disease-induced pulmonary hypertension: a review of pathophysiology, management, and current literature. Pulse. 2021;9(3–4):57–63. doi:10.1159/000519101
15. Fonseca GHH, Souza R, Salemi VMC, Jardim CVP, Gualandro SFM. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012;39(1):112–118. doi:10.1183/09031936.00134410
16. Klings ES, Machado RF, Barst RJ, et al. An official american thoracic society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am J Respir Crit Care Med. 2014;189(6):727–740. doi:10.1164/rccm.201401-0065ST
17. Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34(6):1219–1263. doi:10.1183/09031936.00139009
18. Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–D41. doi:10.1016/j.jacc.2013.10.029
19. Humbert M, Montani D, Evgenov OV, Simonneau G. Definition and classification of pulmonary hypertension. Handb Exp Pharmacol. 2013;218:3–29.
20. Gordeuk VR, Castro OL, Machado RF. Pathophysiology and treatment of pulmonary hypertension in sickle cell disease. Blood. 2016;127(7):820–828. doi:10.1182/blood-2015-08-618561
21. Morris CR, Suh JH, Hagar W, et al. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood. 2008;111(1):402–410. doi:10.1182/blood-2007-04-081703
22. Antzelevitch C, Burashnikov A. Overview of basic mechanisms of cardiac arrhythmia. Card Electrophysiol Clin. 2011;3(1):23–45. doi:10.1016/j.ccep.2010.10.012
23. Ataga KI, Sood N, De Gent G, et al. Pulmonary hypertension in sickle cell disease. Am J Med. 2004;117(9):665–669. doi:10.1016/j.amjmed.2004.03.034
24. Aleem A, Jehangir A, Owais M, et al. Echocardiographic abnormalities in adolescent and adult Saudi patients with sickle cell disease. Saudi Med J. 2007;28(7):1072–1075.
25. Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307(12):1254. doi:10.1001/jama.2012.358
26. Ramphul K, Ramphul Y, Verma R, Kumar N, Joynauth J. Cardiac arrhythmias during sickle cell disease crisis in the United States. Am J Cardiol. 2021;149:160–161. doi:10.1016/j.amjcard.2021.03.030
27. Kapoor A, Thakkar S, Battel L, et al. The prevalence and impact of arrhythmias in hospitalized patients with sickle cell disorders: a large database analysis. Blood. 2020;136(Supplement 1):5–6. doi:10.1182/blood-2020-142099
28. Galiè N, Humbert M, Vachiery JL, et al. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37(1):67–119. doi:10.1093/eurheartj/ehv317
29. Hemnes AR, Forfia PR, Champion HC. Assessment of pulmonary vasculature and right heart by invasive haemodynamics and echocardiography. Int J Clin Pract. 2009;63:4–19. doi:10.1111/j.1742-1241.2009.02110.x
30. Savale L, Habibi A, Lionnet F, et al. Clinical phenotypes and outcomes of precapillary pulmonary hypertension of sickle cell disease. Eur Respir J. 2019;54(6):1900585. doi:10.1183/13993003.00585-2019
31. Ziyadah MS, Mansory EM, Alahwal HM, et al. Predisposing factors and incidence of venous thromboembolism among hospitalized patients with sickle cell disease. J Clin Med. 2023;12(20):6498. doi:10.3390/jcm12206498
32. Anthi A, Machado RF, Jison ML, et al. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175(12):1272–1279. doi:10.1164/rccm.200610-1498OC
33. Sachdev V, Rosing DR, Thein SL. Cardiovascular complications of sickle cell disease. Trends Cardiovasc Med. 2021;31(3):187–193. doi:10.1016/j.tcm.2020.02.002
34. Bolignano D, Rastelli S, Agarwal R, et al. Pulmonary Hypertension in CKD. Am J Kidney Dis. 2013;61(4):612–622. doi:10.1053/j.ajkd.2012.07.029
35. Sutton LL, Castro O, Cross DJ, Spencer JE, Lewis JF. Pulmonary hypertension in sickle cell disease. Am J Cardiol. 1994;74(6):626–628. doi:10.1016/0002-9149(94)90760-9
36. Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101(4):1257–1261. doi:10.1182/blood-2002-03-0948
37. Patel U, Desai R, Hanna B, et al. Sickle cell disease‐associated arrhythmias and in‐hospital outcomes: insights from the National Inpatient Sample. J Arrhythm. 2020;36(6):1068–1073. doi:10.1002/joa3.12418
38. Chaurasia SP, Manvar R. Study of electrocardiographic and echocardiographic changes in sickle cell anaemia patients.
39. Akgül F, Seyfeli E, Melek İ, et al. Increased QT dispersion in sickle cell disease: effect of pulmonary hypertension. Acta Haematol. 2007;118(1):1–6. doi:10.1159/000100929
40. Roberts PR, Green D. Arrhythmias in chronic kidney disease. Heart. 2011;97(9):766–773. doi:10.1136/hrt.2010.208587
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
