Back to Journals » Risk Management and Healthcare Policy » Volume 16
Knowledge, Attitudes and Practices Assessment on Bat-Borne Zoonotic Diseases Among the People of Moyamba District, Sierra Leone
Authors Williams SMT , Ansumana R, Johnny J, Bakarr IA, Osborne A
Received 6 April 2023
Accepted for publication 11 July 2023
Published 19 July 2023 Volume 2023:16 Pages 1331—1342
DOI https://doi.org/10.2147/RMHP.S413802
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Samuel Maxwell Tom Williams,1 Rashid Ansumana,2 Jonathan Johnny,3 Ibrahim A Bakarr,3 Augustus Osborne1
1Department of Biological Sciences, School of Environmental Sciences, Njala University, Freetown, Sierra Leone; 2Department of Public Health, School of Community Health Sciences, Njala University, Freetown, Sierra Leone; 3Department of Wildlife Management and Conservation, School of Natural Resources Management, Njala University, Freetown, Sierra Leone
Correspondence: Samuel Maxwell Tom Williams, Tel +23278462858, Email [email protected]
Background: Bats are considered wildlife species of public health concern, as they are known to host various pathogenic agents, and their interactions with humans are potential routes of pathogen spillover. A high level of knowledge on Bat-borne Zoonotic Diseases (BZD), their causative agents, signs, symptoms, mode and pattern of transmission, health attitudes, and practices towards the disorders are vital parameters in handling them. This study aimed to look into BZD knowledge, public attitudes, and behaviour.
Methods: We surveyed the 14 chiefdoms of Moyamba district. A total of 421 participants were randomly sampled using closed-ended questionnaire. Simple linear regression analysis was used to determine the effects of gender, age, education, and livelihood opportunities on BZD knowledge (at 95% confidence interval and alpha value = 0.05). The findings were analysed and correlated with a scientific and public health perspective to assess the breadth of knowledge and awareness of BZD among the people of Moyamba district.
Results: The findings from the study show a low level of knowledge on BZD among the people of the Moyamba district, with only 119 (28.3%) individuals that had some knowledge about BZD. Of those that knew about BZD, 94 (79.0%) had very little knowledge, 24 (20.2%) had a fair amount, and 1 (0.8%) had a great deal of knowledge about BZD. The primary mode of knowledge dissemination was through social media platforms.
Conclusion: The level of knowledge about BZD is also very low. As a result of these findings, policymakers, health professionals, and environmental educators will be compelled to develop strategies to reduce the risk of BZD transmission in Sierra Leone’s population.
Keywords: bat-borne zoonotic diseases, public health, transmission, Moyamba District, knowledge assessment, policymakers
Introduction
Bat-borne zoonotic diseases (BZD) are diseases spread by bats to human populations via human-bat interactions.1 Wildlife populations are a major source of zoonotic pathogens, and bats are one of the species of public health concern1–4 as they host various pathogenic viruses like; Marburg virus (MARV),5 Ebola virus (EBOV), Nipah virus6,7 etc. Global travel, trade, agricultural development, deforestation, and urbanization have all been linked to increased zoonotic disease spillover, increasing the interface and rate of contact between humans, household animals, and wildlife populations.8,9 Spillover of zoonotic pathogens into human populations occurs through various mechanisms, such as hunting and preparing bats, bites, scratches, contact with bodily fluids, and aerosolization of salvia droplets.7
Several studies have been conducted in Sierra Leone to assess the knowledge, attitudes, and practices of people regarding bat-borne zoonotic diseases. Fasina et al10 found that 70% of participants believed bats were involved in the transmission of EVD, but only 25% knew that washing hands frequently with soap and water could prevent EVD. Euren et al11 found that the consumption of bat meat was a significant risk factor for the transmission of zoonotic diseases, but only 5% knew that consuming bat meat could transmit zoonotic diseases. Finally, Subramanian12 surveyed 123 bushmeat hunters and traders in rural Sierra Leone to investigate hunting practices and awareness of zoonotic disease risk associated with the bush meat trade. The study revealed a 24% were aware of the possibility of disease transmission from animals to humans.
Most of the studies conducted in Sierra Leone focused on bat meat as a risk factor for zoonotic diseases, but other routes of transmission, such as contact with bat urine and faeces, were not adequately explored. Also, one of the significant gaps is the lack of knowledge regarding the specific bat species that carry zoonotic diseases.
This study aims to assess Sierra Leoneans’ knowledge of bat zoonotic diseases and identify potential gaps that could be filled to reduce the risk of disease transmission. The study’s specific goals were
- To determine the level of knowledge and awareness of bat zoonotic diseases among rural communities in Sierra Leone.
- To assess the attitudes and perceptions of rural communities towards bats and bat zoonotic diseases.
- To identify potential risk factors for exposure to bat-borne diseases in Sierra Leone, including bat hunting and consumption, contact with bat dung, and exposure to bat habitats.
Methods
Study Area
The study was conducted in Moyamba District, southern Sierra Leone. The district is the largest in the southern province comprising 14 chiefdoms (Figure 1), occupying a total area of 6902 km2 (2665 mi2) and has a human population of 318,588.13 The region has hills and caves, tropical rain forest, farm bush, and savannah grasslands. Subsistence crops and livestock farming are important to the population.
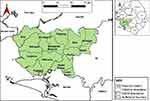 |
Figure 1 Map of Sierra Leone showing Moyamba District and the fourteen chiefdoms (developed by the authors). |
Bats are known to be reservoirs of various zoonotic diseases and Moyamba district has a large population of fruit bats, which increases the risk of exposure to bat-borne zoonotic diseases. Moyamba district, has experienced outbreaks of Lassa fever, Ebola virus disease, and other infectious diseases. There is limited knowledge and awareness about zoonotic diseases and their transmission among the population of Moyamba district. Therefore, it is crucial to assess the level of knowledge among the people of Moyamba district to develop effective strategies to prevent and control bat-borne zoonotic diseases.
Data Collection
The random sampling technique was used in the data collection from the 3rd October 2020 to the 4th March 2021. Individual participants were randomly selected and interviewed across chiefdom level, both in the suburbs/hinterlands and chiefdom headquarters. Because most local have to leave for work (farmers or traders) we only had the chance to interview people we met in their homes across villages. Primary and secondary data were collected for differential perceptions and responses across the 14 chiefdoms of Moyamba district. A household survey method with a closed-ended questionnaire for quantitative analysis using Kobo Toolbox was used for primary data collection (a set of integrated tools for creating forms and collecting interview responses).14
Additionally, qualitative data were collected through in-depth interviews and focus group discussions (FGD) with key stakeholders who were some of the leading agencies involved in post-Ebola response efforts, rabies treatment centres, and individuals interested in the situation of people affected by BZD. The FGDs were typically held with groups of 5–10 participants and the questions or themes were predetermined. Discussions were followed by an assessment of the data to determine the respondents’ knowledge and opinions about bats and their social interactions with humans; knowledge of zoonotic diseases; and knowledge of health practices towards bat-borne zoonoses. Thematic analysis techniques were used to analyze qualitative data.
Sample Size Determination
Males and females aged 10 and up who had lived in the selected areas for at least six months before data collection and were willing to participate in this study were eligible to participate. Using the total population of the Moyamba district (n=318,588) from the 2015 Population and Housing Census,13 we used the Krejcie and Morgan formula15 to estimate a statistically appropriate sample size of 383 respondents with a 95% confidence interval (α=5.0%). We increased the total sample size to 421 respondents to compensate for statistical value
S = required sample size,
X2 = at 95% confidence Level with 1 degree of freedom the chi-square value is X2 = 3.841
N = the population size,
P = population proportion (assume to be 0.5 to provide maximum sample size)
e = the margin of error at 95% Confidence Level e = 0.05
Data Management and Analysis
The survey data were analysed using descriptive statistics such as frequencies and percentages using Microsoft Excel 2019 software. Knowledge about BZD were cross-tabulated with background demographic characteristics of respondents. Simple linear regression analysis was used to determine the effects of gender, age, education, and livelihood opportunities on BZD knowledge (at 95% confidence interval and alpha value = 0.05). Pearson’s Chi squared test was also used to determine the relationship, with a P-value of 0.05 considered significant.
Results
Sociodemographic and Knowledge About Bat-Borne Zoonotic Diseases (BZD)
A total of 421 respondents answered the questionnaire, representing a 100% response rate in the 14 chiefdoms of Moyamba District of Sierra Leone. The study showed that the overall knowledge level regarding BZD is low among the study population, with less than one-third (119/421; 28.3%) of respondents answered to know BZD (Table 1).
 |
Table 1 Knowledge About Bat-Borne Zoonotic Diseases (BZD) (N= 421) |
More males (61.5%; 259/421) than females (162/421; 38.5%) were interviewed, with 1 and 13 males that had a great deal, and fair amount of knowledge about BZD respectively, and 11 females with a fair amount of knowledge about BZD. Furthermore, most respondents (28.3%; 119/421) were between the ages of 30–39, with 25.9% (109/421) between the ages of 20–29 years. Those within the age group 20–29 had a better understanding of BZD (12) people with a fair amount of knowledge about BZD) than the other age groups. Most respondents were illiterate (187/421; 44.4%). However, there were a 24.7% (104/421) people with primary level, 24.2% (102/421) secondary, and 4.5% (19/421) tertiary and 2.1% (9/421) vocational studies (Table 2). Even though fewer tertiary educated people were interviewed, there were more (14) tertiary educated people with a fair knowledge of BZD and only 1 person with secondary education with a great deal of knowledge (Table 2).
 |
Table 2 Cross Tabulation of Sociodemographic Structure of Respondents and Knowledge of Bat-Borne Zoonotic Diseases (BZD). (n = 421) |
Finally, most of the respondents were farmers (48.7%, 205/421) followed by traders (19.2%, 81/421) and the remaining 32.1% is spread across other career opportunities like artisan, fisher, health worker, hunter, teacher and other units of government employments. Even though health workers are one of the fewest people interviewed (3.1%, 13/421), there were more (11) health workers with a fair knowledge of BZD and only 1 person who was a trader with a great deal of knowledge (Table 2).
We used a simple linear regression analysis to see how respondents’ gender, age, and employment opportunities affected their knowledge of BZD. These variables showed a weak positive (age group 20–29 and people with secondary level) and negative (Males and health workers) correlations with respect to their level of knowledge on BZD. However, educational level has a strong effect (p < 0.001) on knowledge about BZD while age has but a weak effect (p = 0.079) on knowledge scores. Furthermore, the simple linear regression model explained 54.1% of the variance observed (R = 0.541), which was a sufficient predictor power, and the ANOVA p-value (p<0.001), indicated a significant modeling. The Pearson Chi Square analysis indicated that, education and livelihood opportunities had a strong (p<0.001) an association with knowledge about BZD (Table 3).
 |
Table 3 Simple Linear Regression on Knowledge About BZD |
In an exclusive analysis (from those that knew about BZD (119 respondents)); 47.1% (56/119) identified viruses only, 9.2% (11/119) identified virus and bacteria only, 4.2% (5/119) identified virus, bacteria and fungi only, and 0.8% (1/119) identified only fungi and virus as bat-borne pathogens. In contrast, 38.7% (46/119) did not know the pathogens that causes BZD (Table 4).
 |
Table 4 Types of Pathogens Carried by Bats; How Infection Occurs; Source of Knowledge Obtained. (Multiple Choice Questions) |
Health Attitudes and Practices Towards Bat-Borne Zoonotic Diseases
Most respondents who knew BZD also had good health practices for BZD outbreaks. 96.6% (115/199) frequently washed their hands with soap, 99.2% (118/119) used face masks, 94.1% (112/119) adhered to government health authorities and policies, 82.4% (98/199) avoided sick people or health workers, 90.8%, (108/119) cleaned their homes and 87.4%, (104/119) maintained social distancing during BZD outbreaks. However, 51.3% (61/119) used public mass transport, 60.5% (72/119) were socially active, ate road snacks and 67.2% (80/119) shared food with strangers during BZD outbreaks (Table 5).
 |
Table 5 Health Attitudes and Practices Towards Bat-Borne Zoonotic Diseases (BZD) |
Socio-Cultural Importance of Bats in the Study Area
Few respondents, 1.4% (6/421) believe bats can bring bed bugs to a home, 9.7% (41/421) said bats can be possessed and used for witchcraft manipulation and 0.2% (1/421) believes brings good omens while amore considerable sum of 88.6% (373/421) believes bats are not attached to any traditional beliefs. Medically, 0.7% (3/421) of respondents use bats to treat asthma disease (Table 6). Moreover, 14.0% (59/421) of the respondents eat bat meat, and 46.3% (195/421) eat food (fruits) partially eaten by bats.
 |
Table 6 Traditional or Cultural Beliefs and Uses of Bats |
Findings from the Focus Group Discussions
A total of 210 respondents were interviewed, with 15 respondents from each of the 14 chiefdoms.
In the focus group discussions, the most common diseases caused by bats strongly mentioned by the respondents are; cholera, coronavirus, diarrhea, ebolavirus, and cheilitis (mouth sores). These diseases were transmitted by eating contaminated food by bats, bat meat and interacting with an infected person. Common signs and symptoms of the illnesses were headaches, high fever, diarrhea, vomiting, hemolysis, and fatigue. Standard and generalized preventive and control measures include observing personal and environmental hygiene, reporting suspected individuals to health authorities, adhering to outbreak policy measures, abstaining from bat meat and food contaminated by bats, and observing social distancing. The focus group discussions taught them about BZD from peer groups, social media and community radio, school and learning institutions, religious gatherings and parents and closed-relatives, respectively.
Respondents from 4 chiefdoms engaged in bat hunting. The most frequently hunted bats were fruit bats like H. monstrosus, Epomops sp., R. aegyptiacus, and Epomophorus sp. They purposely hunt bats for consumption and sale at the market. Respondents believed that bats (specifically H. monstrosus (“Turkay” in Mende dialect)) can be used for witchcraft manipulation, and the presence of other bats (unidentified) combined with other mythical signs can indicate a person is about to die. Medically, bats (Epomops species “Tarjey” in Mende dialect) are used to treat asthma, and relatively small numbers from the chiefdoms use bat guano (feaces and urine) as fertilizer. The Moyamba DHMT reported 58 EVD survivors in the entire Moyamba District, even though neither the Chiefdom Headquarters nor the Moyamba DHMT had any data on the number of Ebola Virus Disease deaths.
Discussion
Respondents’ Knowledge of BZD
This study compared the survey results from a scientific and public health perspective to conclude the level of knowledge and awareness of bats and BZD among the residents of Moyamba District. Only 119/421 respondents (28.1%) knew anything about BZD, which is consistent with a related study that examined knowledge, attitudes, and practices related to exposure to bats and rabies in two rural Guatemalan communities where most survey participants (54%) claimed to know little to nothing about rabies.16 Similar studies have been conducted to evaluate COVID-19 knowledge in various African countries, with a mean score of 16.39 out of 23 in Egypt, Uganda, Nigeria, and Tanzania.17,18 A Chi-Square analysis also showed that education and livelihood activities had an association with knowledge on BZD, which is consistent with a similar study done in Egypt and China.17,19 The findings showed that most respondents recognized EVD as the only BZD which could be as a result of the devastating impact of the 2014 EVD outbreak in Sierra Leone, but few identified Nipah, Hendra, coronaviruses, Marburg, Rabies, Histoplasmosis, and Bartonellosis as bat-borne pathogenic agents. Respondents from the FGD named EVD, MVD, and coronavirus disease BZD, consistent with other findings from Rosenberg R.20 and Bengis et al21 that the primary zoonotic diseases associated with bats are rabies, histoplasmosis etc.
Respondents of Moyamba District identified the mouth, eyes, nostrils, and tissue openings/wounds as the main entry points for BZD pathogens. In general, the transmission of BZD can spread through two primary routes: respiratory droplets expelled during sneezing or coughing inhaled by another person, and contact transmission, where it is picked up on surfaces and infects people when they come into contact with their mucous membranes.22,23 Prior studies have shown that aerosolization, bodily fluids, and feces can lead to the transmission of pathogens like norovirus24 and hantavirus,25 similarly, Ebola and Marburg viruses. The first patients of the EVD outbreak in Sierra Leone had eaten or handled sick or deceased non-human primates or other mammals infected with the EBOV virus, and intra-family transmission and hospital-mediated dissemination were linked to the majority of subsequent cases.26 Non-human primate studies on EBOV invasion through oral transmission showed its high lethality. The alleged first human victims of the outbreak in Gabon27 and DRC7 were linked to the consumption of chimps and fruit bats, respectively, leading to human-to-human transmission.
The primary mode of knowledge transmission about BZD was social media, community radio, and peer group discussions. Community radio is the cheapest and most easily accessible mode of communication, offering a platform for regular community messaging and activities. Peer group discussions play a crucial role in the socialization of adolescents, as they serve as a means of transmitting behavioral norms and standards.28
Health Attitudes Towards Bat-Borne Disease Outbreaks
The principal way to reduce infections is hand washing.29 Most respondents follow the study’s ideal hygienic protocols for preventing and controlling BZD outbreaks, such as washing their hands, using face masks, adhering to government health authorities, staying away from sick people, and cleaning their homes and offices. To reduce the risk of spreading BZD outbreaks through person-to-person transmission, standard precautions such as PPE, safe sharps handling and disposal, routine environmental cleaning, and incorporating safe handling procedures are recommended.30,31
Bat Hunting, Traditional Values and Medicinal Values
Bat Hunting
The Moyamba District in Sierra Leone has a high risk of contracting bat zoonosis due to the fact that 14.0% of respondents reported eating bat meat and 46.3% reported eating food that bats had partially consumed. Bats must be handled, butchered, and prepared for consumption as part of hunting, which increases the risk of zoonotic disease spread. A Nipah-like henipavirus outbreak in Cameroon has been linked to bat butchery32 and the trade in bush meat from dead bats has been linked to at least one Ebola outbreak.33 It is alarming that there is bat hunting in the Moyamba district, as the Marburg virus was discovered in Egyptian rousette bats.5
Moreover, 3.6% of participants use bats guano as fertilizer to improve crop growth and production. Guano may contain viral, bacterial, or fungal pathogens, which can cause BZD to humans. Eating bat-meats poses a severe threat to humans, as spats and half-eaten fruit with bat pathogens can be readily consumed by susceptible animals and even humans.34,35
Traditional Beliefs and Practices of Bats
Respondents in a study believed that bats (especially H. monstrosus) could be used for witchcraft manipulation consistent with Allen36 that described bats as nocturnal creatures with elusive habits frequently viewed as evil creatures. H. monstrosus are continental African fruit bats that make a loud honk that resembles other epomophorines.37 Some respondents also identified some bats as a sign of good omen, similar to Low et al38 who reported in their studies that in Malaysia, ethnic Han Chinese believe bat entering a home is a good omen, but if the bat stays and eats fruit there, the owner will suffer a terrible fate.
Medicinal Values
In our study, the respondents traditionally believed that bats, specifically Epomops species, can be used to treat asthma. Similarly, the flat-faced fruit-eating bat (Artibeus planirostris) and the great fruit-eating bat (Artibeus lituratus) are used as animal medicines to treat alcoholism and asthma, respectively.39 The Papyrus Ebers, a 110-page scroll that is approximately 20 meters long and was written in 1500 BC but is thought to have been copied from texts that date to 3400 BC, contains the earliest evidence of the use of bats for medicinal purposes.40 Walker41 also discovered in his research that Pteropus vampyrus is used in some villages outside Medan, Sumatra, to treat breathing disorders.
Types of Bat-Borne Zoonotic Disease Outbreaks in Moyamba District
The Moyamba District DHMT and the CHCs reported no other bat-borne zoonotic disease cases other than EVD and COVID-19. One plausible explanation is that human exposure to these pathogens is infrequent since bat-borne lyssavirus and other zoonoses are so uncommon in Sierra Leone. Only 58 EVD survivors were registered in the Moyamba district DHMT from the past outbreak in Sierra Leone, with some sequelae including depression, eye and ear defects, itchy skin, nausea and vomiting, unexplained tumors, and headaches. Additionally, societal and psycho-social effects continue to cause survivors’ problems, including work and education disruption. Risk factors for typical mental health issues brought on by the EVD outbreak include poor health, life-threatening conditions, and loved one loss. Because there are not enough qualified mental health professionals in the West African nations where EVD outbreaks are occurring, the risks that those affected will experience long-lasting psychological distress and eventually develop psychopathology could be increased.42 There is a need for a follow-up assessment of the survivors, contacts, and relatives even though the psychological distress described in this study may not be a specific psychological disorder.
Conclusion
Overall, the knowledge about BZD was low among the people of the Moyamba district. The possible causes of low level of knowledge about BZD could be high illiteracy among the respondents. There was a correlation between level of education and livelihood activity with knowledge about BZD and the level of education was the key determinant (P<0.001) in the analysis. Furthermore, the primary source of knowledge about BZD is from peer groups, parents, religious gatherings, social media and community radio discussions, etc. The study also identified the social uses of bats for medicinal values to treat the disease like asthma and the use of bats as a sign of good omen also, as the majority of the respondents were farmers, thus the high tendency to interact with bats and other wildlife animals that could lead to pathogen exposure.
A lack of knowledge may immediately impact practice and result in subpar infection control, the spread of pathogens, a delay in diagnosis, and increased morbidity and mortality. Therefore, more work needs to be done to improve. Community education should proceed as usual with a dedication to utilizing all available tactics to support the population’s advancement of knowledge, attitude, and practice. Additionally, healthcare professionals must be updated and trained to improve their ability to recognize, manage, and contain BZD outbreaks.
Data Sharing Statement
Data are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Research ethical approval was obtained from the Directorate of Research and Development, Njala University, Sierra Leone. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was signed by all participants and by parents or guardian for participants under 18 years of age before conducting the interview.
Consent for Publication
All participants gave consent for publication during data collection.
Acknowledgment
Many appreciations to PhD. Brian R. Amman and Tatyana Klimova from the Centers for Disease Control and Prevention, Viral Special Pathogens Branch, USA for their insightful feedback and assistance with developing the manuscript. PhD. Brian R. Amman uses his ecological disease professionalism to interpret the results and developed the manuscript. The authors would also like to appreciate Esther Marie Williams for sharing her skills in livestock disease surveillance in the development of the research questionnaire. Additionally, authors would like to thank Prof. Richard Wadsworth for critically revised the manuscript for intellectual content.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Chomel BB, Belotto A, Meslin FX. Wildlife, exotic pets, and emerging zoonoses. Emerg Infect Dis. 2007;13(1):6–11. doi:10.3201/eid1301.060480
2. Morse SS. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995;1(1):7–15. doi:10.3201/eid0101.950102
3. Calisher CH, Childs JE, Field HE, et al. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006;19(3):531–545. doi:10.1128/CMR.00017-06
4. Smith I, Wang LF. Bats and their virome: an important source of emerging viruses capable of infecting humans. Curr Opin Virol. 2013;3(1):84–91. doi:10.1016/j.coviro.2012.11.006
5. Amman BA, Bird BH, Bakarr IA, et al. Isolation of Angola-like Marburg virus from Egyptian rousette bats from West Africa. Nat Commun. 2020;11:510. doi:10.1038/s41467-020-14327-8
6. Biek R, Walsh PD, Leroy EM, et al. Recent common ancestry of Ebola Zaire virus found in a bat reservoir. PLOS Pathogen. 2006;2:e90. doi:10.1371/journal.ppat.0020090
7. Leroy EM, Epelboin A, Mondonge V, et al. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 2009;9:723–728. doi:10.1089/vbz.2008.0167
8. Daszak P, Cunningham A, Hyatt A. Emerging infectious diseases of wildlife – threats to biodiversity and human health. Sci J. 2000;287:443–448. doi:10.1126/science.287.5452.443
9. Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001;78(2):103–116. doi:10.1016/s0001-706x(00)00179-0
10. Fasina FO, Shittu A, Lazarus D, et al. Transmission dynamics and control of Ebola virus disease outbreak in Nigeria, July to September 2014. Euro Surveill. 2014;19(40):20920. doi:10.2807/1560-7917.es2014.19.40.20920
11. Euren J, Bangura J, Gbakima A, et al. Human interactions with bat populations in Bombali, Sierra Leone. EcoHealth. 2020;17(3):292–301. doi:10.1007/s10393-020-01502-y
12. Subramanian M. Zoonotic disease risk and the bushmeat trade: assessing awareness among hunters and traders in Sierra Leone. EcoHealth. 2012;9(4):471–482. doi:10.1007/s10393-012-0807-1
13. Statistics Sierra Leone. 2015 population and housing census, summary of final result. Statistics Sierra Leone; 2016. Available from: https://www.statstistics.sl/.images/StatisticsSL/.Documents/final-results_-2015_population_and_housing_census.pdf.
14. World Bank.-UNHCR joint data center on forced displacement: enhancing kobo toolbox for data collection and analysis. Available from: https://www.jointdatacenter.org/enhancing-kobotoolbox-for-data-collection-and-analysis/.
15. Krejcie RV, Morgan DW. Determining of sample size for research activities. Educ Psychol Measure. 1970;30:607–610.
16. Moran D, Juliao P, Alvarez-Lindblade KA, et al. knowledge, attitudes and practices regarding rabies and exposure to bats in two rural communities in Guatemala. BMC Res Notes. 2015;8. doi:10.1186/s13104-014-0955-1
17. Abdelhafiz AS, Mohammed Z, Ibrahim ME, et al. Knowledge, perceptions, and attitude of Egyptians towards the novel Coronavirus Disease (COVID-19). J Community Health. 2020;45(5):881–890. doi:10.1007/s10900-020-00827-7
18. Olum R, Chekwech G, Wekha G, et al. Coronavirus disease-2019: knowledge, attitude, and practices of health care workers at Makerere University Teaching Hospitals, Uganda. Front Public Health. 2020;8:181. doi:10.3389/fpubh.2020.00181
19. Zhong B-L, Luo W, H-M L, et al. Knowledge, attitudes, and practices towards COVID-19 among Chinese residents during the rapid rise of the COVID-19 outbreak: a quick online cross-sectional survey. Int J Biol Sci. 2020;16:1745–1752. doi:10.7150/ijbs.45221
20. Rosenberg R. Detecting the emergence of novel, zoonotic viruses pathogenic to humans. Cell Mol Life Sci. 2015;72(6):1115–1125. doi:10.1007/s00018-014-1785-y
21. Bengis RG, Leighton FA, Fischer JR, et al. The role of wildlife in emerging and re-emerging zoonoses. Rev Sci Tech. 2004;23(2):497–511.
22. WHO (World Health Organization). Water, sanitation, hygiene, and waste management for the COVID-19 virus. Available from: https://apps.who.int/iris/bitstream/handle/10665/331499/WHO-2019-nCoV-IPC_WASH-2020.2-eng.pdf?sequence=1&isAllowed=y.
23. Zhang Z, Zhang L, Wang Y. COVID-19 indirect contact transmission through the oral mucosa must not be ignored. J Oral Pathol Med. 2020;49(5):450–451. doi:10.1111/jop.13019
24. Marks PJ, Vipond IB, Regan FM, et al. A school outbreak of Norwalk-like virus: evidence for airborne transmission. Epidemiol Infect. 2003;131:727–736. doi:10.1017/S0950268803008689
25. LeDuc JW. Hantaviruses. In: Evans AS, Kaslow RA, editors. Viral Infections of Humans. Boston, MA: Springer; 1997. doi:10.1007/978-1-4899-0036-4_12
26. Goeijenbier M, van Kampen JJ, Reusken CB, et al. Ebola virus disease: a review on epidemiology, symptoms, treatment and pathogenesis. Netherlands J Med. 2014;72:442–448.
27. Georges-Courbot MC, Sanchez A, Lu CY, et al. Isolation and phylogenetic characterization of Ebola viruses causing different outbreaks in Gabon. Emerg Infect Dis. 1997;3:59–62. doi:10.3201/eid0301.970107
28. Baptista I, Tomé G, Matos MG, et al. Jovens com Saúde - Diálogo com uma geração. Lisboa: Texto; A Escola. 2008:197–214.
29. Centers for Disease Control and Prevention. Handwashing in community settings. https://www.cdc.gov/handwashing/when-how-handwashing.html.
30. Bolyard EA, Tablan OC, Williams WW, et al. Guideline for infection control in health care personnel. Am J Infect Control. 1998;26:289–354. doi:10.1016/S0196-6553(98)80015-1
31. Demmler Gail J. Infectious Diseases Society of America and Centers for disease control: summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev Infect Dis. 1991;13:315–329. doi:10.1093/clinids/13.2.315
32. Pernet O, Schneider BS, Beaty MS, et al. Evidence for henipavirus spillover into human populations in Africa. Nat Commun. 2014;5:5342. doi:10.1038/ncomms6342
33. Lucas A, Kumakamba C, Lange EC, et al. Serology and behavioral perspectives on Ebola virus diseases among bushmeat vendors in Equateur, Democratic Republic of the Congo, after the 2018 outbreak. Open Forum Infect Dis. 2020;7. doi:10.1093/ofid/ofaa295
34. Amman BR, Jones MEB, Sealy TK, et al. Oral shedding of Marburg virus in experimentally infected Egyptian fruit bats (Rousettus Aegyptiacus). J Wildl Dis. 2015;51(1):113–124. doi:10.7589/2014-08-198
35. Amman BR, Schuh AJ, Albariño CG, Towner JS. Marburg virus persistence on fruit as a plausible route of bat to primate filovirus transmission. Viruses. 2021;13(12):2394. doi:10.3390/v13122394
36. Allen GM. Bats, Biology, Behavior and Folklore. Boston: Harvard University Press; 1939:44–63.
37. Kingdom Jonathan. The Kingdom Field Guide to African Mammals. Princeton University Press; 2015:332–544.
38. Low M, Hoong WZ, Shen Z, et al. Bane or Blessing? Reviewing cultural values of bats across the Asia-Pacific region. J Ethnobiol. 2021;41:18–34. doi:10.2993/0278-0771-41.1.18
39. Bryan CP, Smith GE. The Papyrus Ebers. London: Geoffrey Bles; 1930:167.
40. Rego KMC, Zeppelini CG, Lopez LCS, et al. Assessing human-bat interactions around a protected area in northeastern Brazil. J Ethnobiol Ethnomed. 2015;11:80. doi:10.1186/s13002-015-0058-7
41. Walker S. Some informal correspondence on local people’s medicinal uses of fruit bats. Bat Net News Chiropt Conserv Inf Netw South Asia. 2005;6(1):6.
42. Shultz JM, Baingana F, Neria Y. The 2014 Ebola outbreak and mental health: current status and recommended response. J Am Med Assoc. 2015;313(6):567–568. doi:10.1001/jama.2014.17934
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.