Back to Journals » International Journal of General Medicine » Volume 17
The Association of High Lipoprotein(a) Concentration and Risk of Ischaemic Stroke in Atrial Fibrillation Patients
Authors Zhang S, Zhou Y, Wang J, Fu Q, Shen T, Pan G , Luo R, Yang X, Jiang L , Hu H
Received 16 November 2023
Accepted for publication 10 April 2024
Published 8 May 2024 Volume 2024:17 Pages 2001—2009
DOI https://doi.org/10.2147/IJGM.S449400
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Redoy Ranjan
Siyi Zhang,1,2,* Yue Zhou,1,* Jinghui Wang,2 Qingan Fu,1 Tianzhou Shen,2 Guanrui Pan,2 Renfei Luo,1 Xinlei Yang,3 Long Jiang,1 Hui Hu4
1Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, People’s Republic of China; 2Department of Clinical Medicine, Queen Mary School of Nanchang University, Nanchang, Jiangxi Province, People’s Republic of China; 3Department of Biobank Center, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, People’s Republic of China; 4Department of Medical Big Data Center, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Long Jiang, Department of Cardiovascular Medicine, the Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, People’s Republic of China, Tel +86-13767026990, Email [email protected] Hui Hu, Department of Medical Big Data Center, the Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, People’s Republic of China, Tel +86-13576978125, Email [email protected]
Background: Lipoprotein(a) [Lp(a)] is a well-established risk factor for ischaemic stroke (IS). It is unclear whether Lp(a) is associated with IS in patients with atrial fibrillation (AF). The aim of this study is to explore the association between the concentration of Lp(a) and the risk of IS in AF patients, hope to find the potential risk factor for the IS in AF patients.
Methods: This study is a retrospective cohort study. The screened AF patients between January 2017 and July 2021 were matched at 1:1 by the propensity score matching (PSM) method in the Second Affiliated Hospital of Nanchang University. Associations between Lp(a) and ischaemic stroke were analysed using logistic regression models, stratified analysis and sensitivity analysis. Statistical analyses were conducted using IBM SPSS software.
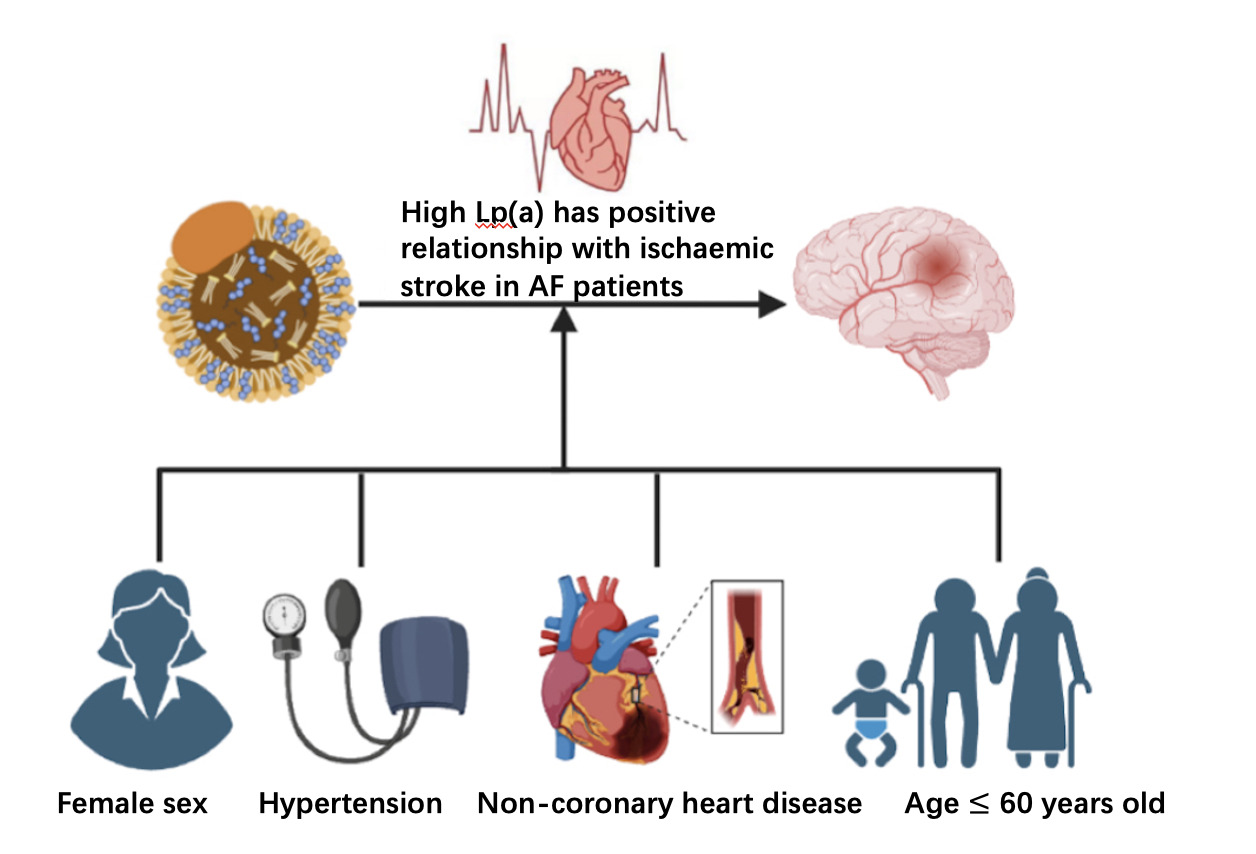
Results: The number of enrolled participates is 2258, which contains 1129 non-AF patients and 1129 AF patients. Among IS patients, the median Lp(a) concentration was higher than that of controls (17.03 vs. 15.36 mg/dL, P = 0.032). The Spearman rank-order correlation coefficients revealed significant positive relationships between IS and Lp(a) (P = 0.032). In addition, a significant increase in IS risk was associated with Lp(a) levels > 30.00 mg/dL in unadjusted model [OR:1.263, 95% CI(1.046– 1.523), P = 0.015], model 1 [OR:1.284, 95% CI(1.062,1.552), P = 0.010], model 2 [OR: 1.297, 95% CI(1.07,1.573). P = 0.008], and model 3 [OR: 1.290, 95% CI (1.064, 1.562). P = 0.009]. The stratified analysis indicated that this correlation was not affected by female sex [1.484 (1.117, 1.972), P = 0.006], age ≤ 60 [1.864 (1.067-3.254), P=0.029], hypertension [1.359 (1.074, 1.721), P = 0.011], or non-coronary heart disease (CHD) [1.388 (1.108, 1.738), P = 0.004].
Conclusion: High levels of Lp(a) were significantly related to IS in AF patients and may be a potential risk factor in the onset of an IS in AF patients.
Keywords: ischaemic stroke, Lipoprotein(a), atrial fibrillation, risk factor, retrospective cohort study
Graphical Abstract:
Introduction
Stroke is a clinical syndrome with high incidence, recurrence, and mortality. It is classified as ischaemic and haemorrhagic stroke. Ischaemic stroke (IS) is caused by decreased blood flow in the brain due to blockage of arteries, resulting in loss of brain cells.1 Incident stroke cases in 2019 numbered 12.2 million, while prevalent strokes numbered 101 million. Therefore, stroke remains a worldwide second most common cause of death, and IS accounts for 62.4% of all incident strokes.2 For patients with atrial fibrillation (AF), the risk of stroke is 5-fold higher, accounting for more than 79% of cardiogenic strokes.3,4 IS caused by AF is more severe, with more complications and poorer prognosis.5 There are various risk factors that influence the incidence of stroke among individuals with AF, including age, hypertension, diabetes, embolism, and ventricular insufficiency.6–8 The guidelines suggest that AF patients with risk factors for IS should be given regular anticoagulant therapy to prevent its occurrence.9
Lipoprotein(a), a low-density lipoprotein cholesterol-like molecule, is bound to apolipoprotein(a). Lp(a) functions against vascular inflammation and atherosclerosis lesions.10 Lp(a) can promote thrombosis by influencing platelets and the coagulation system, resulting in thromboembolism.11,12 ASCVD, including IS, has been linked to high levels of Lp(a).13,14 Lipoprotein(a)-lowering treatments and anticoagulatory drugs are recommended for reduce the risk of ischaemic stroke.15,16
However, the effect of Lp(a) in AF patients is not yet clear since they tend to have lower Lp(a) levels, especially among women.17 Although a high Lp(a) level was confirmed to have a significant relationship with IS, this relationship in AF patients is still controversial.18,19 Therefore, the aim of this study is to explore the relationship between Lp(a) and IS in patients with AF. It is hoped that additional risk factors can be identified for AF patients with IS so that early treatment and prevention of thrombotic events in patients with AF can be achieved.
Methods
Subjects and Groups
Electronic medical records of patients who were hospitalized at the Second Affiliated Hospital of Nanchang University were retrospectively reviewed. Based on a previously published article,17 screened AF patients with or without ischaemic stroke between January 2017 and July 2021 in the Second Affiliated Hospital of Nanchang University were randomly selected based on 1:1 matching for age, sex, smoking, drinking, CHD, and diabetes mellitus (DM) following the principle of the propensity score matching (PSM) method. An institutional review board at the Second Affiliated Hospital of Nanchang University, China, approved this study for ethical reasons. The data are anonymous, and the requirement for informed consent was therefore waived. The study is in line with Declaration of Helsinki.
Clinical Data Collection
A variety of general information was collected, including age, sex, smoking, alcohol consumption, systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index, CHD, and DM. Recorded laboratory data included levels of total cholesterol (TC), total triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), non-HDL-C, apolipoprotein A-I, apolipoprotein B, Lp(a), albumin, alkaline phosphatase, homocysteine (HCY), uric acid, creatinine, eGFR, fasting plasma glucose (FPG), high-sensitivity C-reactive protein (hsCRP), platelet count, neutrophil count, neutrophil ratio, lymphocyte count, lymphocyte ratio, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and pharmacological treatments including Clopidogrel, Statin, Aspirin, Fibrates, calcium channel blockers (CCB), angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB), and Beta-blocker.
Relevant Definitions
Human serum Lp(a) concentrations were determined using the Lp(a) Assay Kit (Latex Enhanced Immunoturbidimetric Method, Beijingantu Inc., China) after fasting for over 8 hours. Two Lp(a) Assay Kits (Shanghai Kehua Biology Inc., China, production batch: 20180212 and Beijing Antu Inc., China, LOT:10723C11) were used.20 Monoclonal antibodies KIV-8 and KIV-9 reacts with Lp(a) on the latex granules using latex immunoturbidimetric method. The reaction absorbance was correlated with the concentration of serum Lp(a). Lp(a) concentrations normally range from 0 to 3000 mg/dL.17 Coronary heart disease (CHD) is diagnosed if at least one coronary lumen stenosis ≥50% is confirmed by coronary angiography (CAG).21 Hypertension is diagnosed until repeated SBP > 140 mmHg and/or DBP > 90 mmHg (at least 3 times).22 The diagnosis of AF was made on the basis of the electrocardiogram (ECG). The diagnostic criteria for AF were the disappearance of normal P waves and the appearance of irregular RR intervals.23,24 Strokes may be categorized into two types, ischemic, or haemorrhagic, by computed tomography (CT), or by magnetic resonance imaging (MRI). Ischaemic strokes were diagnosed when a brain CT or MRI showed an abrupt blockage of an artery or no evidence of haemorrhage.25 For this analysis, we only analysed ischaemic stroke.
Statistical Analysis
Statistical analysis of collected data was performed using SPSS version 26.0 (SPSS, Inc., Chicago, Illinois). The figure was made using R (version 4.5.0). In order to make research subjects clinically comparable, the PSM method balances covariates and reduces bias between the IS group and control group. For each object, PSM builds a regression model that estimates an object’s propensity score of 0 to 1. Propensity scores represent the likelihood of the subject being assigned to the IS group. As a result, balances in the distributions of covariate between the IS and control groups were made using PSM. To compare clinical data between two groups, the Mann–Whitney U-test was applied to continuous variables presented as medians and the chi-square test was applied to categorical variables presented as frequencies and percentages (%). To estimate the magnitude and direction of the monotonic associations between IS and other factors, Spearman rank-order correlation coefficients were used. Lp(a) concentrations were divided into two groups: 0–30 mg/dL and >30 mg/dL. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated using logistic regression models. No factors were adjusted for in the unadjusted model; model 1 was adjusted for SBP and LDL-C; while model 2 was further adjusted for Hcy, NLR, and Apolipoprotein A-I plus model 1. As the pharmacological treatments may affect the level of serum lipids, we conducted logistic regression model 3 adjusted for model 2 plus Fibrates, CCB, ACEI and ARB, and Beta-blocker.15 As the Lp(a) contains a part of LDL-C, we conducted another sensitivity analysis in order to observe whether the corrected LDL-C will affect the results. The binary regression analyses after adjusting LDL-C for Lp(a) were carried out by using the following formula: adjusted LDL-C = LDL-C (mmol/L) – [Lp(a) (mg/dL) x 0.0002586×0.173 (or 0.30, or 0.45)].22 Furthermore, stratified analyses were performed for gender (men and women) age (≤60 and >60 years), with or without hypertension, and with or without CHD. The statistical significance level in our study was set at P < 0.05.
Result
Clinical Characteristics
In total, 1129 patients with ischaemic stroke and 1129 controls matched for age, sex, smoking, drinking, CHD, and DM by the PSM method were randomly selected. Baseline characteristics are shown in Table 1. Briefly, the median age was 73 years old after matching. Moreover, the group of patients with ischemic stroke had higher DBP (87 vs 83 mmHg, P = 0.001) and SBP (143 vs 139 mmHg, P = 0.001). In addition, the distribution of Lp(a) and the proportions of both control and ischaemic stroke groups are skew distribution (Figure S1), and the levels of Lp(a) were significantly elevated in patients with ischaemic stroke (17.03 vs 15.36 mg/dL, P = 0.032). The platelet count (178 vs 170 × 10^9/L, P = 0.016), neutrophil count (4.05 vs 3.8 × 10^9/L, P < 0.0001), neutrophil ratio (67.4 vs 66.2, P = 0.007), and NLR (2.94 vs 2.74, P = 0.005) were significantly higher in patients with ischaemic stroke. However, the lymphocyte ratio was higher in non-ischaemic stroke patients (22.7 vs 24.2, P = 0.005). Statin (920 vs 447, P < 0.001) and Aspirin (465 vs 214, P < 0.001) were much higher in ischaemic stroke group.
 |
Table 1 Baseline Characteristics of Ischemic Stroke Cases and Controls in AF Patients After Matching |
Correlations Between Ischaemic Stroke and Other Clinical Indicators
The Spearman rank-order correlation coefficients showed that there were significant positive relationships between ischaemic stroke and Lp(a) (r: 0.045*), DBP (r: 0.102**), SBP (r: 0.096**), and NLR (r: 0.059**) (Table S1). In addition to ischaemic stroke, the Spearman rank-order correlation coefficients also indicated that there were significantly positive relationships between Lp(a) and DBP (r: 0.049*), SBP (r: 0.053*), TC (r: 0.101**), LDL-c (r: 0.126**), nonHDL (r: 0.107**), apoB (r: 0.153**), creatinine (r: 0.057**), CRP (r: 0.092**), fibrinogen (r: 0.160**), NLR (r: 0.099**), and PLR (r: 0.103**) (Table S2). There were significantly positive relationships between Lp(a) and Clopidogrel (r: 0.067**), Statin (r: 0.069**), and Aspirin (r: 0.058**) (Table S2). While there was a negative relationship between Lp(a) and Fibrate (r: −0.025) (Table S2). There was a significant but negative relationship between Lp(a) and albumin (r: −0.067**) and eGFR (r: −0.065**) (Table S2).
The Relationship Between Lp(a) and Ischaemic Stroke
According to the value of Lp(a) concentrations, two groups were divided: group 1: 0–30.00 mg/dL; group 2: >30.00 mg/dL. The binary conditional logistic regression was applied to determine if the Lp concentration independently correlated with IS risk in patients with AF.
Using an unadjusted model, Lp(a) > 30 mg/dl had a significant positive association with IS [OR: 1.263, 95% CI (1.046–1.523), P = 0.015] (Table 2). After adjusting for SBP, and LDLC in model 1, the result was similar, showing a significant positive relationship between Lp(a) > 30 mg/dl and IS [OR: 1.286, 95% CI (1.064–1.554), P = 0.009] (Table 2). In addition, after adjustment for the factors contained in the model 1 plus apolipoprotein A-I, Hcy, and NLR, it was found that IS was positively correlated with Lp (a) in AF patients [OR: 1.283, 95% CI (1.061–1.552). P = 0.010] (Table 2). In model 3, after additional adjustment of model 2 plus Fibrates, CCB, ACEI and ARB, and Beta-blocker, Lp(a) still had positive association with IS in AF patients [OR: 1.290, 95% CI (1.064, 1.562). P = 0.009] (Table 2). In model 4, after additional adjustment of model 2 plus Statin, the relationship between Lp(a) and ischaemic stroke in AF patients was not significant [OR: 1.133, 95% CI (0.919–1.396). P = 0.241] (Table S3).
 |
Table 2 Odd Ratios (95% Confidence Intervals) for Ischemic Stroke and Lp(a) |
In sensitive stratified analysis after adjusting LDL-C for Lp(a), Lp(a) > 30 mg/dl had a significant positive association with IS in unadjusted model [OR: 1.245, 95% CI (1.031–1.505), P = 0.023]. After adjusting for SBP and LDLC, the association was significant positive [OR: 1.221, 95% CI (1.009–1.477), P = 0.040]. Moreover, after adjusting for the factors included in model 1 plus apolipoprotein A-I, Hcy, and NLR, the correlation was not statistically significant [OR: 1.196, 95% CI (0.985–1.452), P = 0.070]. The stratified analysis revealed correlation associations between IS and Lp(a) adjusting for LDL-C are significant with smaller LDL-C adjustments (Table S4).
In the stratified analysis, Lp(a)>30 mg/dL leads to a significantly higher risk of IS in female [OR:1.459, 95% CI(1.100–1.934), P = 0.009] (Figure 1A), patients with hypertension [OR:1.337, 95% CI(1.059–1.688), P = 0.015](Figure 1B), non-CHD patients [OR:1.356, 95% CI(1.086–1.692), P = 0.007] (Figure 1C), and patients with age ≤ 60 years old [OR:1.864, 95%CI(1.067-3.254), P=0.029] (Figure 1D).
 |
Figure 1 Odd ratios (95% confidence intervals) for ischemic stroke of Lp(a) groups by Gender (A), Hypertension (B), CHD (C) and Age (D). |
Discussion
In this retrospective cohort study, we found a significantly positive relationship between Lp(a) > 30 mg/dl and ischaemic stroke in AF patients, particularly in women and age ≤ 60 years old. Moreover, AF patients with hypertension without CHD have a notable positive correlation between their Lp(a) levels and the occurrence of ischemic stroke.
The relationship between Lp(a) and IS has been reported in recent years. However, current studies on the connection between Lp(a) level and risk of ischaemic stroke in AF patients are few and controversial. A cross-sectional study revealed that patients with nonvalvular atrial fibrillation who were in the highest quartile of Lp(a) levels were 1.23 times more likely to experience IS than those in the lowest quartile (OR, 1.23; 95% CI, 1.04–1.45).18 On the other hand, the community-based ARIC (Atherosclerosis Risk in Communities) cohort study showed a 42% increased risk of ischaemic stroke associated with high Lp(a) (≥50 mg/dl) only in patients without AF.19 Moreover, in a Mendelian randomization study, the authors also discovered no causal link between Lp(a) with AF and ischaemic stroke.26 This study used PSM method to minimize potential bias and used sensitive stratified analysis to further analysis, the results suggested that there was a positive relationship between high Lp(a) and IS in AF patients.
After careful matching for age, sex, smoking, drinking, CHD, and DM by the PSM method, our study confirmed that high Lp(a) levels (>30 mg/dL) were significantly positively associated with ischaemic stroke in AF patients. The potential mechanism may include the following: (1) AF can decrease the blood flow rate and increase the risk of thrombosis, causing an elevated chance of blocking cerebral blood vessels due to the easier detachment of thrombi.27 (2) Lp(a) is considered a risk factor for developing atherosclerotic cardiovascular diseases because of its relevance to atherogenesis, thrombosis, and inflammation.28 Therefore, although there are lower median Lp(a) levels in AF patients, those with higher Lp(a) levels may still have a greater risk of developing ischaemic stroke.
This study found a substantial positive correlation between Lp(a) and IS, particularly in female AF patients. This is consistent with other studies, such as Ohria et al, who showed a significant relationship between high Lp(a) levels and ischaemic stroke risk in women but not in men (black women: RR = 1.84, 95% CI, 1.05–3.07, white women: RR = 2.42, 95% CI, 1.30–4.53).29 Studies have found that the levels of Lp(a) in females are increased after menopause.30–32 The sample in our study was aged from 28 to 98 years; however, the median age was 73 years. Ageing women experience hormonal changes due to menopause. Hormonal changes affect metabolism in fat synthesis, fat accumulation, and energy consumption.33 For example, oestrogen loss may increase visceral fat and reduce LDL receptor activity, causing β-oxidation decreases and dyslipidaemia.34 This study also showed that the median Lp(a) concentration in women was much higher than that in men (female, 16.28 mg/dL; male, 15.96 mg/dL), which is the same as previous studies. The increased incidence of dyslipidaemia in menopause and the greater Lp(a) concentration in women may cause atherosclerosis, which leads to ischaemic stroke. Therefore, females are much more likely than males to show a significant positive association between Lp(a), and higher ischaemic stroke risk.
There were studies reported that different treatments may affect the concentration of Lp(a).15,35,36 Our study did not found that the impact of medication on the correlation between Lp(a) and IS except for statins. Because this is a case-control study, all patients with IS (previous diagnosed or newly diagnosed) were included which cause most of patients with IS have already treated with statins (the baseline showed 81% vs 39%). The high rate of statins in baseline would influence the results of logistic regression. In addition, many studies found that long-term of statins have no effect on or slightly increase the concentration of Lp(a).35,37 Therefore, it suggested that the use of medication would not affect the correlation between Lp(a) and IS in AF patients. Furthermore, this significant association between Lp(a) and IS was found only in patients with hypertension and without CHD. Studies have found that CHD is a risk factor for ischaemic stroke in AF patients.38 However, in patients with CHD, there was no apparent relationship between Lp(a) and ischaemic stroke. There were three possible reasons. First, CHD was matched by PSM, and the median Lp(a) levels between patients with CHD and patients without CHD were similar (16.20 mg/dL vs 16.12 mg/dL, P = 0.383). Second, the total number of CHD patients was too small (391 vs 161), which made it difficult to detect significant differences. Third, studies have confirmed that high Lp(a) levels have a positive relationship with CHD. Therefore, the association between Lp(a) and ischaemic stroke may be decreased in the CHD population.
In the hypertension subgroup, there were 553 non-hypertension patients with high Lp(a) levels and 198 non-hypertension patients with low Lp(a) levels. The unbalanced intergroup differences and the small sample size may have contributed resulted in the unachieved significant differences. On the other hand, the blood vessel endothelia in patients with hypertension are damaged, especially in uncontrolled hypertension patients, which leads to internal inflammation and atherosclerosis.39 High levels of plasma Lp(a) will also contribute to atherosclerosis.40,41 The combination of the two can easily cause thrombus detachment, leading to the occurrence of ischaemic stroke. However, further confirmation of the current results is required through prospective studies.
Study Strength and Limitations
This study used PSM and sensitive stratified analysis methods to find that high concentration of Lp(a) is associated with ischaemic stroke in AF patients. The findings provided new points for the stroke risk assessment in patients with AF. There are some limitations in our study. (1) As a retrospective cohort study, only the relationship between Lp(a) and the risk of ischaemic stroke was assessed, but not the causal relationship. (2) This study relies on historical records, which may have potential bias. Only parts of confounding factors are analyzed which may interfere the results. (3) Since Lp(a) levels are determined genetically, the subtypes of Lp(a) should be included in further studies. (4) The unit of Lp(a) was not mmol/L, it could be better than mg/dl.
Conclusion
Our study found that there is a positive correlation between Lp(a) and the risk of ischaemic stroke in AF patients. This suggests that high Lp(a) should be a potential risk factor for ischaemic stroke in AF patients. For those patients with moderate to high stroke risk, Lp(a) should be measured at least once in their lifespan. Lp(a)-lowing therapies may have benefit for preventing the ischaemic stroke in AF patients with high Lp(a) levels. However, these results should be confirmed by a well-designed prospective cohort study.
Acknowledgment
Siyi Zhang and Yue Zhou are co-first authors for this study. We thank Extreme Smart Analysis platform (https://www.xsmartanalysis.com/) for its analysis assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Key R&D Program of China (No.2022YFE0209900, 2021YFC2500600, and 2021YFC2500602), and Jiangxi Province 03 Special Project and China Mobile Jiangxi Company’s 5G Construction and Application Demonstration Project (No. 20212ABC03A37). Key R&D Program (general projects) of Jiangxi Provincial Department of Science and Technology (No. 20202BBGL73040, 20202BBEL53005). Applied research and cultivation program of Jiangxi Provincial Department of Science and Technology (No. 20212BAG70012).
Disclosure
The authors declare that there is no conflict of interest in this work.
References
1. Walter K. What is acute ischemic stroke? JAMA. 2022;327(9):885. doi:10.1001/jama.2022.1420
2. Feigin VL, Stark BA, Johnson CO, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. doi:10.1016/S1474-4422(21)00252-0
3. Fohtung RB, Rich MW. Identification of patients at risk of stroke from atrial fibrillation. US Cardiol Rev. 2016;10(2):60–64. doi:10.15420/usc.2016:1:1
4. Pujadas Capmany R, Arboix A, Casañas-Muñoz R, Anguera-Ferrando N. Specific cardiac disorders in 402 consecutive patients with ischaemic cardioembolic stroke. Int J Cardiol. 2004;95(2–3):129–134 doi:10.1016/j.ijcard.2003.02.007.
5. Glader E-L, Sjölander M, Eriksson M, Lundberg M. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke. 2010;41(2):397–401. doi:10.1161/STROKEAHA.109.566950
6. Lip GYH, Edwards SJ. Stroke prevention with aspirin, warfarin and ximelagatran in patients with non-valvular atrial fibrillation: a systematic review and meta-analysis. Thromb Res. 2006;118(3):321–333 doi:10.1016/j.thromres.2005.08.007.
7. Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120–129. doi:10.1056/NEJMoa1105575
8. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867 doi:10.7326/0003-4819-146-12-200706190-00007.
9. Baman JR, Passman RS. Atrial Fibrillation. JAMA. 2021;325(21):2218. doi:10.1001/jama.2020.23700
10. Lampsas S, Xenou M, Oikonomou E, et al. Lipoprotein(a) in atherosclerotic diseases: from pathophysiology to diagnosis and treatment. Molecules. 2023;28(3). doi:10.3390/molecules28030969
11. Ouweneel AB, Van Eck M. Lipoproteins as modulators of atherothrombosis: from endothelial function to primary and secondary coagulation. Vascul Pharmacol. 2016;82:1–10. doi:10.1016/j.vph.2015.10.009
12. Deguchi H, Elias DJ, Griffin JH. Minor plasma lipids modulate clotting factor activities and may affect thrombosis risk. Res Pract Thromb Haemost. 2017;1(1):93–102 doi:10.1002/rth2.12017.
13. Langsted A, Nordestgaard BG, Kamstrup PR. Elevated Lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol. 2019;74(1):54–66. doi:10.1016/j.jacc.2019.03.524
14. Hoogeveen RC, Ballantyne CM. Residual cardiovascular risk at low LDL: remnants, Lipoprotein(a), and inflammation. Clin Chem. 2021;67(1):143–153. doi:10.1093/clinchem/hvaa252
15. Di Fusco SA, Arca M, Scicchitano P, et al. Lipoprotein(a): a risk factor for atherosclerosis and an emerging therapeutic target. Heart. 2022;109(1):18–25. doi:10.1136/heartjnl-2021-320708
16. Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet. 1989;1(8631):175–179 doi:10.1016/s0140-6736(89)91200-2.
17. Tao J, Yang X, Qiu Q, et al. Low lipoprotein(a) concentration is associated with atrial fibrillation: a large retrospective cohort study. Lipids Health Dis. 2022;21(1):119. doi:10.1186/s12944-022-01728-5
18. Song J, Zhang X, Wei M, Bo Y, Zhou X, Tang B. Association between lipoprotein(a) and thromboembolism in patients with non-valvular atrial fibrillation: a cross-sectional study. Lipids Health Dis. 2022;21(1):78. doi:10.1186/s12944-022-01682-2
19. Aronis KN, Zhao D, Hoogeveen RC, et al. Associations of Lipoprotein(a) levels with incident atrial fibrillation and ischemic stroke: the ARIC (Atherosclerosis Risk in Communities) Study. J Am Heart Assoc. 2017;6(12):e007372 doi:10.1161/JAHA.117.007372.
20. Fu Q, Hu L, Xu Y, Yi Y, Jiang L. High lipoprotein(a) concentrations are associated with lower type 2 diabetes risk in the Chinese Han population: a large retrospective cohort study. Lipids Health Dis. 2021;20(1):76. doi:10.1186/s12944-021-01504-x
21. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. doi:10.1161/CIR.0b013e31826e1058
22. Unger T, Borghi C, Charchar F, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi:10.1161/HYPERTENSIONAHA.120.15026
23. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi:10.1093/eurheartj/ehaa612
24. Karakayali M, Artac I, Omar T, Rencuzogullari I, Karabag Y, Hamideyin S. Assessment of the efficacy of the electrocardiographic P-wave peak time in predicting atrial high rate episode in patients with cardiac implantable electronic devices. J Electrocardiol. 2023;80:40–44. doi:10.1016/j.jelectrocard.2023.05.001
25. Lin MP, Liebeskind DS. Imaging of ischemic stroke. Continuum. 2016;22(5):1399–1423 doi:10.1212/CON.0000000000000376.
26. Wang S, Zha L, Chen J, et al. The relationship between lipoprotein(a) and risk of cardiovascular disease: a Mendelian randomization analysis. Eur J Med Res. 2022;27(1):211. doi:10.1186/s40001-022-00825-6
27. Escudero-Martínez I, Morales-Caba L, Segura, T. Atrial fibrillation and stroke: A review and new insights. Trends Cardiovasc Med. 2023;33(1):23–29. doi:10.1016/j.tcm.2021.12.001
28. Reyes-Soffer G, Ginsberg HN, Berglund L, et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42(1):e48–e60. doi:10.1161/ATV.0000000000000147
29. Ohira T, Schreiner PJ, Morrisett JD, Chambless LE, Rosamond WD, Folsom AR. Lipoprotein(a) and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2006;37(6):1407–1412 doi:10.1161/01.STR.0000222666.21482.b6.
30. Simony SB, Mortensen MB, Langsted A, Afzal S, Kamstrup PR, Nordestgaard BG. Sex differences of lipoprotein(a) levels and associated risk of morbidity and mortality by age: the Copenhagen General Population Study. Atherosclerosis. 2022;355:76–82. doi:10.1016/j.atherosclerosis.2022.06.1023
31. Aljawini N, Aldakhil LO, Habib SS. High-risk Lipoprotein(a) levels in Saudi women and its relationship to menopause and adiposity. Nutrients. 2023;15(3):693 doi:10.3390/nu15030693.
32. Liu S-L, N-q W, Guo Y-L, et al. Lipoprotein(a) and coronary artery disease in Chinese postmenopausal female patients: a large cross-sectional cohort study. Postgrad Med J. 2019;95(1128):534–540. doi:10.1136/postgradmedj-2019-136591
33. S-h K, Jung Y. Energy metabolism changes and dysregulated lipid metabolism in postmenopausal women. Nutrients. 2021;13(12):4556 doi:10.3390/nu13124556.
34. Middelberg RPS, Spector TD, Swaminathan R, Snieder H. Genetic and environmental influences on lipids, lipoproteins, and apolipoproteins: effects of menopause. Arterioscler Thromb Vasc Biol. 2002;22(7):1142–1147 doi:10.1161/01.atv.0000022889.85440.79.
35. Tsushima T, Tsushima Y, Sullivan C, Hatipoglu B. Lipoprotein(a) and atherosclerotic cardiovascular disease, the impact of available lipid-lowering medications on Lipoprotein(a): an update on new therapies. Endocr Pract. 2023;29(6):491–497. doi:10.1016/j.eprac.2022.12.011
36. Duarte Lau F, Giugliano RP. Lipoprotein(a) and its significance in cardiovascular disease: a review. JAMA Cardiol. 2022;7(7):760–769. doi:10.1001/jamacardio.2022.0987
37. Tsimikas S, Gordts P, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41(24):2275–2284. doi:10.1093/eurheartj/ehz310
38. Chen TY, Uppuluri A, Aftab O, Zarbin M, Agi N, Bhagat N. Risk factors for ischemic cerebral stroke in patients with acute amaurosis fugax. Can J Ophthalmol. 2022. doi:10.1016/j.jcjo.2022.10.010
39. Johansson BB. Hypertension mechanisms causing stroke. Clin Exp Pharmacol Physiol. 1999;26(7):563–565 doi:10.1046/j.1440-1681.1999.03081.x.
40. Zheng KH, Arsenault BJ, Kaiser Y, et al. apoB/apoA-I Ratio and Lp(a) associations with aortic valve stenosis incidence: insights from the EPIC-Norfolk Prospective Population Study. J Am Heart Assoc. 2019;8(16):e013020. doi:10.1161/JAHA.119.013020
41. Sawabe M, Tanaka N, Nakahara K, et al. High lipoprotein(a) level promotes both coronary atherosclerosis and myocardial infarction: a path analysis using a large number of autopsy cases. Heart. 2009;95(24):1997–2002. doi:10.1136/hrt.2008.160879
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
